Revised: 8 December 2025
Information for Industry
Categorisation of Products
Ingredients
Purpose for use
Other sources of information
Categorisation guidance
Excluded products
Requesting a Product Categorisation
Definitions
We have prepared this information to help those wishing to market products understand the factors that determine the category under which a product is regulated. It provides guidance in determining whether a product is a therapeutic product, and if so, whether it is medicine, a medical device or a related product.
The categorisation of a product is determined by its ingredients, its purpose for use and the manner in which it is presented in the market.
Ingredients
Products marketed as dietary supplements, supplemented foods, cosmetics, or related products are not permitted to contain ingredients scheduled as:
- controlled drugs under the Misuse of Drugs Act 1975, or
- prescription medicines, restricted (pharmacist-only) medicines or pharmacy-only medicines under the Medicines Act 1981.
Check Medsafe's Classification database to determine if an ingredient is scheduled under the Medicines Act. When searching for a substance in the schedule, remember to check synonyms if the initial search gives a "not found" result.
The Schedules at the end of the Misuse of Drugs Act contain the lists of controlled drugs.
The Cosmetic Product Group Standard includes lists of substances, with conditions, that can be included in cosmetics. These do not include substances that are scheduled as controlled drugs under the Misuse of Drugs Act 1975 or scheduled as prescription medicines, restricted (pharmacist-only) medicines or pharmacy-only medicines under the Medicines Act 1981. The Environmental Protection Authority (EPA) administers the Cosmetic Product Group Standard.
Purpose for use
A fundamental consideration is whether you intend your product to have a therapeutic purpose. The Medicines Act has a broad definition for therapeutic purpose.
If you intend to have a therapeutic purpose for your product, it will be categorised as a medical device, a medicine or related product.
The purpose for use may be stated or implied in any of the following:
- the product label statements and claims
- websites
- depictions and context of advertising. For example, advertising depicting a pharmacist selling a product to a patient could suggest it has a therapeutic purpose
- education sessions
- testimonials
- provision of, references to or links to information about past or present traditional use
- social media posts and use of influencer-generated material
- television, radio, digital and print media
- the product meets the definition of a medicine in the Medicines Act
- the expected use of the product.
Therapeutic claims are not permitted for products supplied as dietary supplements, supplemented foods or cosmetics.
You can seek independent advice on whether a claim implies a therapeutic purpose.
- The Association of New Zealand Advertisers offers a Therapeutic Advertising Pre-vetting Service (TAPS).
- There are a number of independent regulatory consultants in New Zealand who specialise in advertising compliance.
Other sources of information
Distributors wishing to import unprocessed plant or animal material should contact NZ Biosecurity to determine which import standards apply.
The New Zealand Customs Service can advise on the requirements for commercial importation.
Categorisation guidance
Figure 1 is a guide to help determine the appropriate regulatory coverage for a product. Please note that the legal definition for a medical device in New Zealand may be different to that in other countries. Therefore, even when a product has been accepted as a medical device in other international jurisdictions, it is important to check that it meets the definition of medical device in the Medicines Act 1981 before it is supplied and notified to WAND. Refer to the Definitions section below.
Table 1 provides categorisation information for various types of products as determined when the definitions were amended 1 July 2014. The examples shown in the table are product types that lie close to the medicine / medical device interface.
Figure 1: Flowchart to determine the appropriate regulatory coverage for a product
See the Definitions section for an explanation of the terms in the flowchart
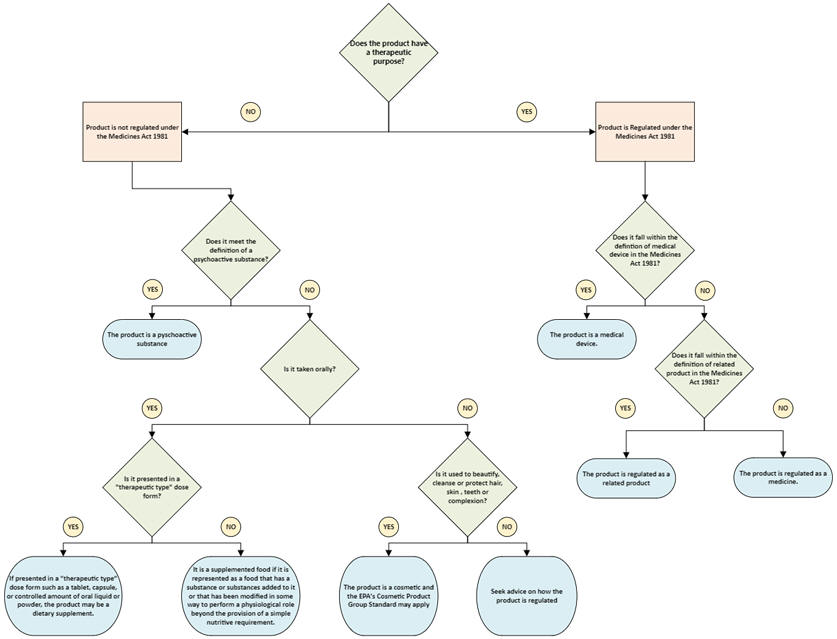
View larger version (pdf, 1 page 122 KB)
Table 1: Categorisation information by product type
(listed in alphabetical order)
| Product type | Category |
|---|---|
| Absorbable intra-ocular and synovial visco-elastic fluids used in surgery | Medical device |
| Artificial tears or saliva | Medical device |
| Blood bags with / without anticoagulant / preservative | Medical device |
| Bone cement with antibiotic | Medical device |
| Catheter with heparin / antibiotic coating | Medical device |
| Combination pack which includes one or more approved medicines in an approved package, with or without a medical device, related product or cosmetic (eg, approved head lice treatment in its approved package combined with a combing conditioner and a comb). | Medicine |
| Contact lens lubricants and solutions | Medical device |
| Condoms with spermicide / viricide / local anaesthetic | Medical device |
| Cryogenic liquids for use with an applicator for cryogenic therapy, where the mode of action is freezing resulting from evaporation | Medical device |
| Dental cement with antibiotic / adrenaline | Medical device |
| Dermal fillers (eg, collagen injections) with / without local anaesthetic included in the formulation | Medical device |
| Douches for body "cleaning" | Medical device |
| Haemodialysis solutions | Medical device |
| Haemostatic agents – where the only action is absorption of fluid | Medical device |
| Haemostatic agents – fibrin | Medicine |
| Hormone eluting intra-uterine contraceptive devices | Medicine |
| Injectable contrast agents for use in diagnostic imaging (eg, CAT, NMR, X-Ray, ultrasound) | Medicine |
| Intra-uterine contraceptive devices other than hormone eluting IUCDs (includes copper containing IUCDs) | Medical device |
| In-vitro pregnancy tests | Medical device |
| Irradiating apparatus | Medical device |
| Lubricating gels without local anaesthetic included in the formulation | Medical device |
| Manuka honey dressings provided the action of the honey is not described as being an antibiotic / antibacterial | Medical device |
| Medicated dressings where the primary purpose of the dressing is to cover and protect the wound and provide an environment that supports healing (in contrast to being a delivery mechanism for a medicine). | Medical device |
| Peritoneal dialysis solutions and substances | Medicine |
| Procedure kits (no medicines included) | Medical device |
| Procedure kits which include an approved medicine in its original pack | Medical device |
| Saline nasal sprays | Medical device |
| Saline for injection | Medicine |
| Solutions for irrigation | Medical device |
| Tamponade solutions for eye surgery | Medical device |
| Tissue adhesives (including fibrin based) | Medical device |
Toothpastes for sensitive teeth where the mode of action
is physical (eg, by blocking open pores).
|
Medical device |
| Total Parenteral Nutrition (TPN) solutions | Medicine |
| Transdermal patches | Medicine |
| Vascular balloons with / without medicinal coating | Medical device |
| Urea ointment for nail debridement | Medical device |
| Ultrasonic therapy apparatus | Medical device |
| Water for injection | Medicine |
Products that are excluded from regulation under the Medicines legislation
Section 58A of the Medicines Regulations 1984 excludes some products from regulation as therapeutic products if certain conditions are met, including limits on the therapeutic claims that can be made.
The excluded products include some dentifrices, some anti-dandruff hair products, some anti-acne skin care products, some barrier creams, and some anti-bacterial skin products. See Table 2 for more information.
Table 2: Types of products and the conditions to be met in order for those products to be excluded from regulation under the Medicines Act 1981
| Conditions | Notes |
|---|---|
| Oral hygiene products (eg, toothpaste, mouthwash) | |
| The product must not contain a substance which, in the amount
or concentration present, is a scheduled medicine. The claimed benefits from use of the product relate only to:
The claimed benefits do not refer to the prevention or treatment of tooth sensitivity. |
The labelling or promotional material must not refer to other
benefits from use of the product, such as prevention or treatment
of tooth sensitivity, gum disease or other oral disease or periodontal
conditions. The products must comply with the requirements specified in the Cosmetic Product Group Standard administered by EPA. |
| Anti-dandruff hair care products (eg, shampoos, conditioners) | |
| The product must not contain a substance which, in the amount
or concentration present, is a scheduled medicine. The claimed benefits from use of the product relate only to the prevention or control of dandruff through cleansing, moisturising, exfoliating or drying the scalp. |
The labelling or promotional material must not refer to other
benefits from use of the product, such as prevention or treatment
of fungal infections of the scalp. The products must comply with the requirements specified in the Cosmetic Product Group Standard administered by EPA. |
| Anti-acne skin care products (eg, cleansers, face scrubs and masks, spot treatments) | |
| The product must not contain a substance which, in the amount
or concentration present, is a scheduled medicine. The claimed benefits from use of the product relate only to the prevention or control of acne through cleansing, moisturising, exfoliating or drying the skin. |
|
| Barrier creams (eg, hand creams, nappy rash creams) | |
| The product must not contain a substance which, in the amount
or concentration present, is a scheduled medicine. The claimed benefits from use of the product relate only to the prevention or control of skin irritation, dryness or damage, by the creation of a protective barrier to moisture. |
The labelling or promotional material must not refer to other
benefits from use of the product, such as prevention or treatment
of fungal or bacterial skin infections. The products must comply with any requirements specified in the Cosmetic Product Group Standard administered by EPA. |
| Antibacterial skin products | |
All of the following conditions must be
met for the exclusion to apply:
The product is a medicine if any one or more of the statements listed above applies to the product. |
The product is not recommended for use in connection with
the provision of health services (as defined in
section 2 of the Health and Disability Commissioner Act
1994). The excluded products must comply with the requirements specified in the Cosmetic Product Group Standard administered by EPA. |
Requesting a Product Categorisation from Medsafe
If you need assistance to determine whether a product is a medicine or a medical device, you can submit a request to Medsafe for the categorisation status of the product.
Please provide the following information with the request.
- Name and contact details of the importer/supplier in NZ
- Name and location details of the manufacturer
- Name of the product
- A description of the purpose of the product
- Information about the mode(s) of action of the product
- A description of the form of the product
- A description of the ingredients in the product (ingredient name and strength)
- Information about any regulatory approvals the product has from
other regulators or notified bodies including:
- the name of the regulator/notified body
- the type of approval granted (CE Mark, FDA 510k, FDA Pre Market Assessment, TGA Registration or Inclusion, etc)
- A copy of the promotional material for the product
- URL of a website detailing the product
- A copy of the product label
Medsafe may also request further information about the product to make the categorisation decision.
Please submit the above information to Medsafe by email: askmedsafe@health.govt.nz
Definitions
Therapeutic purpose
Therapeutic purpose - means any of the following purposes, or a purpose in connection with any of the following purposes:
- preventing, diagnosing, monitoring, alleviating, treating, curing, or compensating for, a disease, ailment, defect, or injury; or
- influencing, inhibiting, or modifying a physiological process; or
- testing the susceptibility of persons to a disease or ailment; or
- influencing, controlling, or preventing conception; or
- testing for pregnancy; or
- investigating, replacing, or modifying parts of the human anatomy
Medicine
- means any substance or article that -
- is manufactured, imported, sold, or supplied wholly or principally for administering to 1 or more human beings for a therapeutic purpose; and
- achieves, or is likely to achieve, its principal intended action in or on the human body by pharmacological, immunological, or metabolic means; and
- includes any substance or article -
- that is manufactured, imported, sold, or supplied wholly or principally for use as a therapeutically active ingredient in the preparation of any substance or article that falls within paragraph (a); or
- of a kind or belonging to a class that is declared by regulations to be a medicine for the purposes of the Medicines Act; but
- does not include -
- a medical device; or
- any food within the meaning of section 2 of the Food Act 1981; or
- any radioactive material within the meaning of section 5(1) of the Radiation Safety Act 2016; or
- any animal food in which a medicine (within the meaning of paragraph (a) or (b)) is incorporated; or
- any animal remedy; or
- any substance or article of a kind or belonging to a class that is declared by regulations not to be a medicine for the purposes of the Medicines Act.
Medical device
- means any device, instrument, apparatus, appliance, or other
article that -
- is intended to be used in, on, or for human beings for a therapeutic purpose; and
- does not achieve its principal intended action in or on the human body by pharmacological, immunological, or metabolic means (but may be assisted in its function by such means); and
- includes a material that -
- is intended to be used in or on human beings for a therapeutic purpose; and
- does not achieve its principal intended action in or on the human body by pharmacological, immunological, or metabolic means (but may be assisted in its function by such means); and
- also includes -
- anything that is intended to be used with a device, instrument, apparatus, appliance, article, or material referred to in paragraph (a) or (b) to enable the device, instrument, apparatus, appliance, article, or material to be used as its manufacturer intends; and
- any device, instrument, apparatus, appliance, article, or material of a kind or belonging to a class that is declared by regulations to be a medical device for the purposes of the Medicines Act; but
- does not include a device, instrument, apparatus, appliance, article, or material of a kind or belonging to a class that is declared by regulations not to be a medical device for the purposes of the Medicines Act.
Related product
A related product is defined in section 94 of the Medicines Act 1981. A related product is a cosmetic or dentifrice or food in respect of which a claim is made that the substance or article is effective for a therapeutic purpose. It does not include any medicine.
A product that is used "wholly or principally" for a therapeutic purpose is a medicine. A related product has a therapeutic purpose that is not its principal purpose (eg, a fluoride toothpaste, where the principal purpose is to clean the teeth).
Many products at the food-therapeutic product interface are likely to be related products (eg, a capsule containing vitamins and minerals where the principal purpose is to supplement the dietary intake of those substances).
Supplemented food
A product is considered a supplemented food if it is represented as a food that has a substance or substances added to it or that has been modified in some way to perform a physiological role beyond the provision of nutritive requirement.
Supplemented foods are regulated under the Food Act 2014 and are subject to the NZ Food (Supplemented Food) Standards 2010.
Dietary supplement
Dietary supplements are regulated under the Food Act 2014 and are subject to the Dietary Supplements Regulations 1985 (administered by Medsafe). These regulations specify a number of requirements for dietary supplements relating to matters such as composition, labelling and maximum permitted daily doses for many vitamins and minerals.
Cosmetic
A product is a cosmetic if it is used to beautify, cleanse or protect the hair, skin, teeth or complexion.
Refer to the Cosmetic Product Group Standard, published by the Environmental Protection Authority (EPA) under the Hazardous Substances and New Organisms (HSNO) legislation. This group standard includes lists of chemicals whose use in cosmetics is restricted.
Refer also to regulations 22, 24 and 26-36 of the Medicines Regulations 1984 for requirements that apply to cosmetics.
"Therapeutic type" dose form
A therapeutic type dose form is a presentation of the product in a form generally used in pharmaceuticals such as tablets, capsules and controlled amounts of oral liquids or powders.
Psychoactive substances
Psychoactive substances are regulated under the Psychoactive Substances Act 2013. This act contains the definition of psychoactive substances.
See the Psychoactive substances regulation page on the Ministry of Health website for more information.





