Revised: 2 May 2013
Safety Information
Update on Pradaxa (dabigatran etexilate)
Medsafe notes that there have been a number of New Zealand publications discussing the safety and use of dabigatran (Pradaxa) in the last few weeks.
This update provides information on the suspected adverse reaction (ADR) reports submitted to the Centre for Adverse Reactions Monitoring (CARM) since dabigatran was approved in New Zealand.
Since the intensive media interest in dabigatran in 2011, the number of ADR reports submitted per month has fallen (Figure 1).
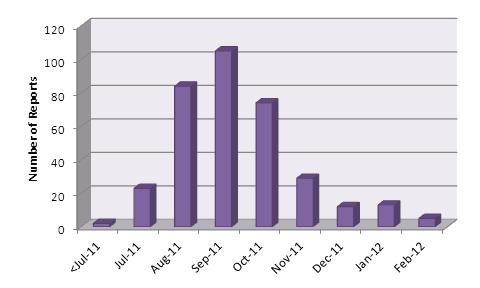
Figure 1: Number of suspected adverse reaction reports received by CARM per month
The number of serious reports* (89) received to the end of February 2012 equates to 26% of all reports (n=345).
Overall CARM considers that these reports may be categorised into four main themes, see Table 1 (note: one report may appear in more than one category).
Table 1: Overview of types of suspected adverse reactions reported
| Grouping | Number of Reports | % of Total Reports (n=345) |
|---|---|---|
| Bleeding | 139 | 40.3 |
| GI Symptoms (non-haemorrhagic) | 145 | 42.9 |
| Thromboembolic | 14 | 4.1 |
| Events secondary to inappropriate use | 28 | 8.1 |
These proportions have remained relatively stable since funding of dabigatran began.
There has been considerable interest in the types of patients experiencing adverse reactions1. A breakdown of the reports received by CARM up to February 2012, by patient age is shown in Figure 2.
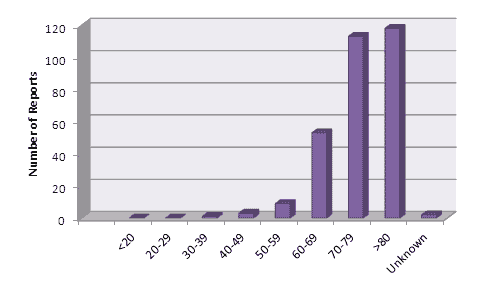
Figure 2: Age of patients experiencing suspected adverse reactions to dabigatran reported to CARM up to the end of February 2012.
Since usage data for New Zealand has now been published the number of reports per age group can be compared with the number of exposed patients (Figure 3).
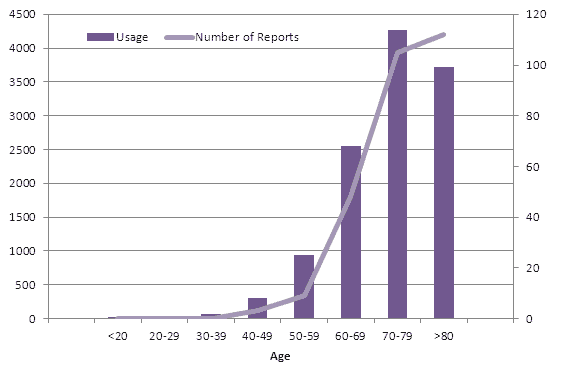
Figure 3: Comparison of number of exposed patients and reported suspected adverse reactions up to the end of December 2012. The usage data and number of reports displayed is for the period July 2011 until December 2011. The usage data was taken from PHARMAC data in The New Zealand Medical Journal, 9 March 2012 (p101)2. The number of reports was taken from CARM data. Please note that unknown age (n=2) was excluded from this graph, as were the two ADRs reported previous to the six month usage period (one from 2009 and one from 2010).
As seen in Figure 3, the number of ADR reports in each age group mimics the pattern of dabigatran usage, except for the over eighty group.
Medsafe recommends careful monitoring of patients aged 80 years and above who are taking dabigatran, particularly those with impaired renal function or low body weight. A reduced dose of dabigatran is recommended for the prevention of stroke in patients with atrial fibrillation for those considered at risk of bleeding (consider 110mg twice daily).
References
- Bell S, Nand J, Spriggs D, et al. 2012. Initial experience with dabigatran etexilate at Auckland City Hospital. The New Zealand Medical Journal, 125(1349): 105-107. Retrieved from http://journal.nzma.org.nz/journal/125-1349/5064/
- Metcalfe S, Moodie P. 2012. National prescribing data for dabigatran. The New Zealand Medical Journal, 125(1351): 97-105. Retrieved from http://journal.nzma.org.nz/journal/125-1351/5112/
*Includes reports where the patient was noted to have died, been hospitalised, required intervention, the events were life threatening or the patient experienced persistent disability





