Revised: 1 January 2024
Medicines
Medsafe's Policy on Electronic Submissions
The intention of this guidance is to ensure that electronic dossiers
submitted to Medsafe can be clearly identified
with the associated application
or notification, and readily accessed, navigated and manipulated as necessary
to facilitate evaluation of the application and supporting data.
Requirements for Different Application Types
High-Risk applications
Medsafe requires all high-risk New Medicine Applications (NMAs) to be submitted with two copies of an electronic dossier in addition to one hard copy of the dossier. This is to allow the clinical and pharmaceutical chemistry evaluators to simultaneously evaluate the application.
All other applications
Applicants are encouraged to submit two copies of an electronic dossier, in addition to the hard copy dossier, for all low-risk and intermediate-risk NMAs. Applicants should also consider electronic dossiers for Changed Medicine Notifications (CMNs) that contain substantial supporting data.
Inclusion of two copies of an electronic dossier will be mandatory for all over-the-counter (OTC) medicine submissions from 1 September 2013. This requirement is outlined in the staged implementation of the OTC reform process and is required to support the future introduction of a single entry portal for lodgement of applications.
Submission Process
One hard copy of modules 1 to 5, including a complete table of contents for each module, is typically required. However, modules 4 and 5 can be submitted in electronic format only if they contain a large number of volumes.
A hard copy of the contents pages to modules 4 and 5 must be provided when submitting these modules solely in electronic format.
Overseas evaluation reports, and associated correspondence, submitted as part of the abbreviated evaluation process must be provided in hard copy
Two copies of the electronic dossier should be provided on CD or DVD in the required format (see below). Medsafe cannot accept electronic dossiers on a data stick, hard drive, rewriteable disk or similar media. Before accepting the electronic dossier, it will be checked for compatibility with Medsafe systems. A replacement copy may be requested at any point during the duration of consent.
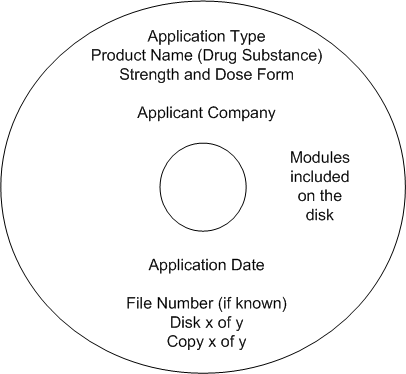
The physical disks should be clearly labelled with the following:
- application type
- product name (drug substance)
- strength and dose form
- applicant company
- application date
- file number (if known)
- disk and copy number
- modules included on the disk.
A suggested format is graphically represented below.
If a sticker is used to label the disk it must not impact on the ability for the disk to be read. An additional label is not required on the CD or DVD case.
Electronic dossiers should be packaged carefully and securely to ensure they arrive in a usable condition. In Medsafe's experience a courier bag by itself does not provide adequate protection for shipping. If the electronic dossier is being provided separately from the hard copy dossier it should be sent in a secure package with an accompanying letter; loose disks are not appropriate.
As with all applications submitted to Medsafe, applicants submitting electronic dossiers must provide an assurance that the content of the electronic copies is identical to that of the paper copy. They must also commit to holding a complete hard copy of the data set for the duration of consent and for a period not less than five years from the date approval lapses. The complete data set may be requested at any time.
Formatting of Electronic Submissions
Medsafe does not require dossiers to be prepared with eCTD software or in NeeS format, but documents in electronic format should:
- be in pdf except the application form which may be in MS Word
- have files and folders structured to correspond with CTD format
- be readable in Acrobat Reader version X (10)
- enable the user to easily view a clear and legible copy of the information
- enable the user to print each document page by page maintaining fonts, orientation, formats and page numbers
- include a well-structured table of contents with hyperlinks to sections
- allow information (including images) to be copied and pasted into other common programmes
- contain hyperlinks and bookmarks to cross-referenced information
- virus checked using up to date programmes (with confirmation of this to be provided in the cover letter)
- not have any security settings or password protection enabled.
Organisation of any electronic response to a request for information (RFI) should follow the same principles as the initial electronic submission. However, responses can be aligned with the questions (as currently occurs in hard copy responses) rather than be structured to correspond with CTD format.
Source of Electronic Document
It is preferable that dossiers are created from an electronic source document so they can be searched and copied to other documents.
Scanned paper documents are inferior to those produced from an electronic source document as they are more difficult to read and do not allow search function capability. If scanning is essential then optical character recognition software (OCR) is required and text verified as accurate prior to submission. Scanning must be at resolutions to ensure the document is legible on screen and when printed. As a guide, text documents should be scanned at 300 dpi and photographs at 600 dpi.
The following documents do not need to be converted to searchable text.
- GMP certificates.
- Certificates of analysis.
- Manufacturer's licences.
- Certificates of suitability.
- Documents in foreign languages and for which a translation is provided as searchable text.
- Literature references (expect those in bibliographic applications).
- Hand-written documents such as batch records and operating logs.
This guidance document is based on Providing Regulatory Submissions in Electronic Format - General Considerations issued by the US Food and Drug Administration.
For complete details refer to the FDA guidance (PDF 54.3 KB, 17 pages).





