Revised: 2 November 2022
About Medsafe
See also Investigation and Enforcement Team
What is Medsafe?
Medsafe is the New Zealand Medicines and Medical Devices Safety Authority. It is a business unit of the Ministry of Health and is the authority responsible for the regulation of therapeutic products in New Zealand.
Medsafe's mission
is
To enhance the health of New Zealanders
by regulating medicines and medical devices
to maximise safety and benefit.
Medsafe has around 60 staff operating out of two offices, with centralised administrative functions, product approval and standard setting based at the head office in Wellington. Investigation and Border Control functions are based in Auckland. Medsafe's management structure is shown in the following organisation chart.
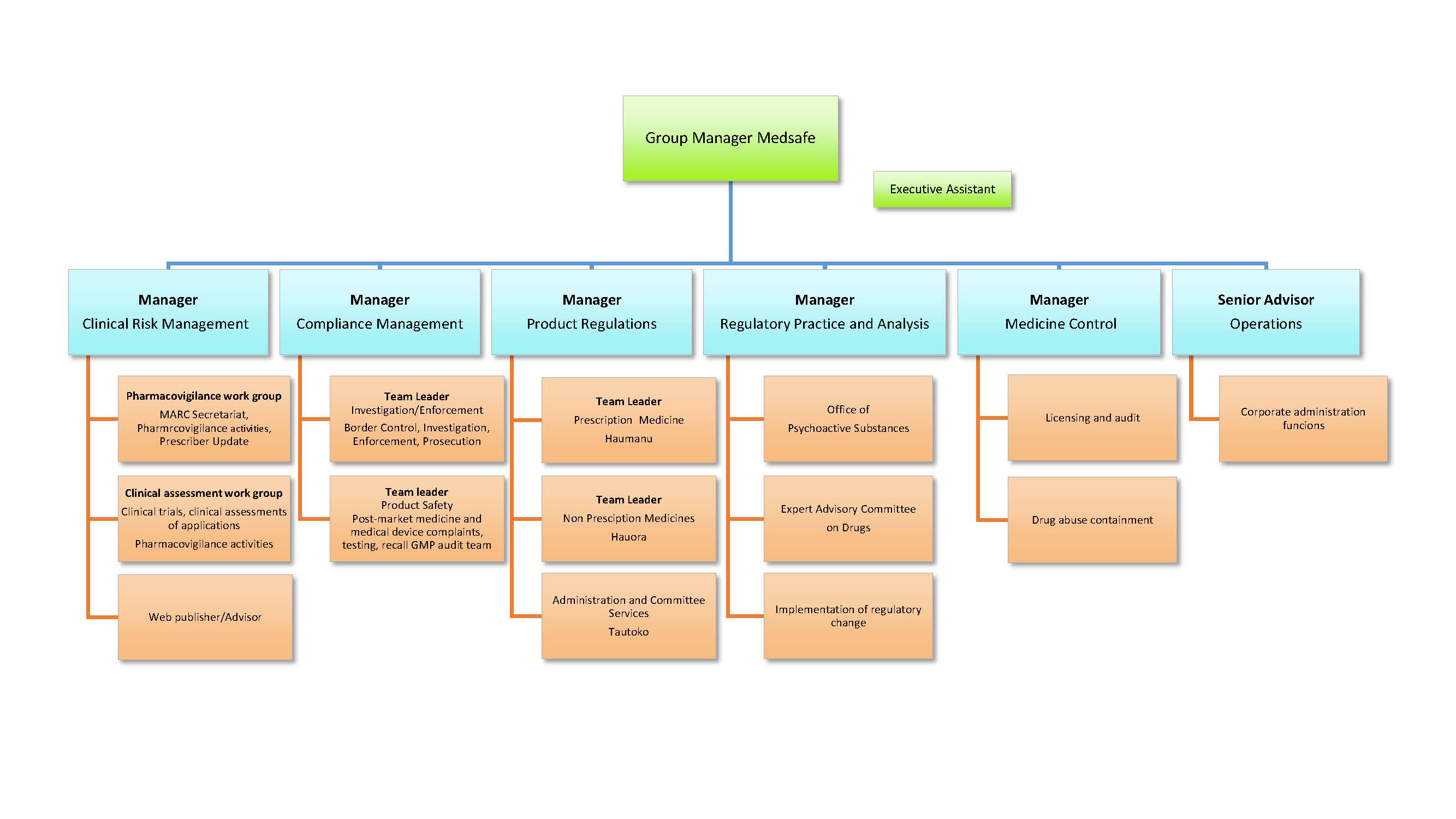
View larger version (pdf, 1 page 225 KB)
In carrying out its functions, Medsafe is accountable to the Ministry of Health, and through the Ministry to the Minister of Health. It is also accountable to the pharmaceutical industry for those activities which are funded by fees collected from the industry.
What We Do
Medsafe is responsible for administering the Medicines Act 1981 and Regulations
1984.
Enquires relating to pharmacy licensing and the Misuse of Drugs Regulations
1977 should be referred to
Medicines Control officers.
What products does Medsafe regulate?
Medsafe regulates products used for a therapeutic purpose. These include:
- medicines
- related products
- medical devices
- controlled drugs used as medicines
"Therapeutic purpose" is defined in Section 4 of the Medicines Act, and includes the treatment, diagnosis and prevention of disease or the modification of a physiological function. It also includes cleaning, soaking or lubricating contact lenses, effecting contraception or inducing anaesthesia.
"Medicine" is defined in Section 3 of the Medicines Act. A product is a medicine if it has a pharmacological effect and is used in humans primarily for a therapeutic purpose.
"Medical device" is defined in Section 3A of the Medicines Act. Medical devices exert their therapeutic effect by physical rather than pharmacological means. The term covers products ranging from wound dressings to heart valves.
For further information about the products regulated by Medsafe and the types of controls used, you should:
- refer to the Medicines legislation; or
- refer to the New Zealand Regulatory Guidelines for Medicines Volume 1.
How are therapeutic products regulated?
The Medicines legislation manages the risk of avoidable harm associated with the use of medicines by ensuring that:
- medicines meet acceptable standards of safety, quality and efficacy;
- personnel, premises and practices used to manufacture, store and distribute medicines comply with requirements designed to ensure that products meet acceptable standards right up until they are delivered to the end-user; and
- information about the selection and safe use of medicines is provided to health professionals and consumers.
Medsafe is responsible for applying a framework of controls designed to ensure that the therapeutic products available in New Zealand are those that can be expected to have greater benefits than risks if used appropriately. This is achieved through:
Pre-marketing approval must be obtained for new and changed medicines. New medicines cannot be marketed in New Zealand without the consent of the Minister of Health. Medicines to which changes have been made cannot be marketed without the consent of the Director-General of Health. Data that satisfactorily establish the quality, safety and efficacy of the product, for the purposes for which it is to be used, must be submitted for evaluation before consent can be granted.
Medsafe is not involved in funding medicines; this is the responsibility of PHARMAC. However, both Medsafe and PHARMAC are committed to facilitating New Zealanders’ access to safe and effective pharmaceuticals – read the Memorandum of Understanding (PDF 218 KB, 8 pages).
Post-marketing surveillance monitors the safety of medicines and medical devices in use. Products shown to be unsafe are removed from use, and prescribers are advised about new safety information for products. Post-marketing surveillance is achieved through activities such as:
- monitoring adverse reactions to medicines used in New Zealand and monitoring the international literature and other information sources;
- testing marketed medicines against product quality standards;
- handling complaints and investigations; and
- auditing and licensing medicine manufacturers.
Vision and Values
In working to achieve our mission, we will:
- apply accepted international practice to the regulation of therapeutic products
- provide timely and unbiased information to health professionals and consumers about the safe use of therapeutic products
- provide fee-paying pharmaceutical industry clients with an efficient service measured against agreed performance targets
- provide courteous and timely responses to written and telephoned enquiries
- prepare and maintain guidelines for the pharmaceutical industry, to assist them in carrying out their responsibilities relating to the manufacture, distribution and use of therapeutic products
- apply processes that are consistent and transparent, and regularly review work processes to improve integrity and efficiency
- maintain a professional approach and a commitment to excellence in all aspects of therapeutic product regulation
- respond in a constructive way to any complaints about, or suggestions for improvements to, our services or activities.
Legislation
Medsafe administers the following legislation.
- The Medicines Act 1981 and Medicines Regulations 1984
For free public access to New Zealand legislation: www.legislation.govt.nz





