Published: 21 July 2021
Safety Information
Alert communication
Myocarditis and pericarditis – rare adverse reactions to Comirnaty (Pfizer COVID-19 vaccine)
21 July 2021
On 9 June 2021 we published a Monitoring Communication on a safety signal of myocarditis with Comirnaty vaccine. It has now been concluded by Medsafe, other national regulators (such as the FDA) and the company, Pfizer, that myocarditis (inflammation of the heart muscle) is a rare side effect of vaccination with Comirnaty. As it is sometimes difficult to distinguish between myocarditis and pericarditis (inflammation of the sac surrounding the heart), the warning will relate to both conditions. This information will be added to the data sheet very shortly.
The wording will be similar to the update recently made in the United Kingdom:
There have been very rare reports of myocarditis and pericarditis occurring after vaccination with Comirnaty often in younger men and shortly after the second dose of the vaccine. These are typically mild cases and individuals tend to recover within a short time following standard treatment and rest.
Healthcare professionals should be alert to the signs and symptoms of myocarditis and pericarditis. Vaccinated individuals should also seek immediate medical attention should they experience new onset of chest pain, shortness of breath, palpitations or arrhythmias. 1
Myocarditis has also been seen in relation to COVID-19 infection.
- Advice for consumers and caregivers
- Information for healthcare professionals
- Product affected
- Further information
- Useful links
Advice for consumers and caregivers
- In the first few days after your vaccination seek medical attention if you experience new onset chest pain, shortness of breath or an abnormal heartbeat. These are potential signs of myocarditis.
- Myocarditis has affected less than one person in a million people who have had Comirnaty vaccine in the European Union countries.3 In most cases the myocarditis was mild and is not expected to have any long-term effects.
- Comirnaty COVID-19 vaccine is highly effective in protecting people from COVID-19 infection, which can also cause myocarditis
- Please report any adverse event following immunisation with COVID-19 vaccine to CARM
Information for healthcare professionals
- Myocarditis has been determined to be a rare side effect (less than 1 in a million overall3) to vaccination with Comirnaty. This information will be added to the data sheet very shortly.
- Be alert to the signs and symptoms of myocarditis and pericarditis occurring in people after vaccination.
- Vaccinated individuals should be advised to seek immediate medical attention should they experience new onset of chest pain, shortness of breath, palpitations or arrhythmias.
- There is no contraindication or change to the vaccination schedule required for the first or second dose of the vaccine for anyone who has experienced myocarditis in the data sheet update. However, a decision to vaccinate should be made with the individual after full consideration of their circumstances.
- Globally, the rate appears to be highest in younger men (aged 18-24 years) and after the second dose. The rate estimated from confirmed cases in health databases in the USA was 8 per million for 12-39 year olds after dose 2 (the estimated rate with the Moderna vaccine was higher in this analysis).4 Myocarditis occurred mostly within 4 days but in a few cases up to 14 days after vaccination.
- To date, CARM and Medsafe have received reports for people across a wide range of ages. Some of the reports of myocarditis were in women and/or occurred after the first dose.
- Myocarditis following Comirnaty vaccination is usually mild, and individuals have tended to recover within a short time following standard treatment and rest.
- Myocarditis after vaccination should be treated as usual. Consult applicable guidance and/or consult specialists (eg, cardiologists) if needed for more information on management.
- Please continue to report adverse events following immunisation with COVID-19 vaccines to CARM
Product affected
| Product name | Sponsor |
|---|---|
| Comirnaty | Pfizer BioNTech |
Further information
Myocarditis is inflammation of the heart muscle, while the tissue forming a sac around the heart is inflamed in pericarditis. Myo-pericarditis means that both the heart muscle and the sac are inflamed.
There are many possible causes of myocarditis, the most common being viral infection. Over 100 people are discharged from hospital with a principle diagnosis of myocarditis in New Zealand every year. It has been shown that men are three times more susceptible to myocarditis than women. Young men (aged 16–20 years) are especially at risk of experiencing myocarditis, while women are affected most after menopause.5 The estimated rate of hospitalisation due to myocarditis in New Zealand is around 2 per 100,000 person years (Interim data SAFE study led by Associate Professor Helen Petousis-Harris, University of Auckland).
Myocarditis has been seen in relation to Covid 19 infection. The short- and long-term implications of SARS CoV-2 infection-induced myocarditis are being monitored.6
The United States was among the first countries starting to vaccinate people from 12 years of age with Comirnaty. Currently the rate estimated from confirmed cases in health databases in the USA was 8 per million for 12-39 year olds after dose 2. The rate was higher at 23 cases per million in 12-39 year olds when unconfirmed cases were included in the estimate (this rate was in males, there were no cases reported in females).4 The overall rate across all ages and both sexes currently remains at less than 1 in a million3. Myocarditis and pericarditis reactions have also been reported for other COVID-19 vaccines, such as Moderna, and to a much lesser extent AstraZeneca and Janssen vaccines.
Up to 19 July 2021, the Centre for Adverse Reactions Monitoring (CARM) has received 9 cases of myocarditis, 5 cases of pericarditis and 4 cases of myopericarditis. Eight of the cases involved male vaccinees. Not all the cases reported to date were admitted to hospital. Cases reported to CARM are being reviewed by the Independent Safety Monitoring Board. To date there has been no clear increase in cases of myocarditis in New Zealand. The age ranges are shown in Figure 1.
Figure 1: Ages of people reported with myocarditis/pericarditis after vaccination in New Zealand
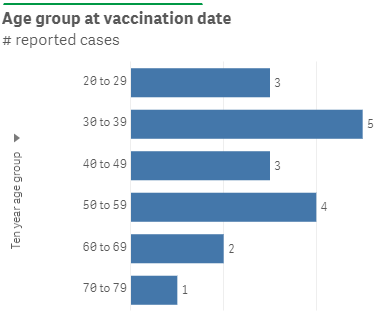
* these reports do not necessarily have a causal relationship with administration of Comirnaty and may represent coincidental events.
Five of the reported reactions occurred after the first dose and 13 occurred after the second dose.
The New Zealand data sheet for Comirnaty will be updated shortly with a myocarditis and pericarditis warning and precaution. Medsafe will continue to monitor this adverse reaction to Comirnaty to ensure we have identified risk factors and outcomes for anyone affected.
Useful links
- Search for consumer medicine information (CMI) and data sheets
- Monitoring communication
- Report an adverse reaction to a medicine
References
- Medicine & Healthcare products Regulatory Agency. 2021. Information for healthcare professionals on COVID-19 vaccine Pfizer/BioNTech (Regulation 174) 9 July 2021. URL: https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/information-for-healthcare-professionals-on-pfizerbiontech-covid-19-vaccine (accessed 19 July 2021).
- Centers for Disease Control and Prevention. 2021. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021. MMWR 70(27): 977–82. URL: https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7027e2-H.pdf (accessed 19 July 2021)
- European Medicines Agency. Comirnaty and Spikevax: possible link to very rare cases of myocarditis and pericarditis 9 July 2021. URL: https://www.ema.europa.eu/en/news/comirnaty-spikevax-possible-link-very-rare-cases-myocarditis-pericarditis (accessed 19 July 2021).
- Shimabukuro, T. 2021. COVID-19 vaccine safety updates 23 June 2021. URL: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVID-Shimabukuro-508.pdf (accessed 19 July 2021).
- Kytö V, Sipilä J, Rautava P. 2013. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. 99(22): 1681–4. DOI: 10.1136/heartjnl-2013-304449 (accessed 19 July 2021).
- Abbasi, J. 2021 Researchers investigate what COVID-19 does to the heart. JAMA 325(9): 808–11. URL: https://jamanetwork.com/journals/jama/fullarticle/2776538 (accessed 19 July 2021).





