Published: September 2012
Publications
Quinolones - A Tendoncy to Rupture
Prescriber Update 33(3): 23-24
September 2012
Tendon rupture and tendinitis are rare adverse events associated with the use of quinolone antibiotics. The Achilles tendon is most frequently involved but other sites can also be affected1.
Although the mechanism for this toxicity is not yet fully understood, a direct toxic effect on collagen fibres has been suggested as occasionally symptoms occur after a single dose of a quinolone2.
Currently, the known risk factors for tendon disorders associated with the use of quinolones include1, 2:
- patients over 60 years of age
- concomitant steroid therapy
- chronic kidney disease
- previous kidney, heart or lung transplant.
Since 2007, there have been a total of 53 reports to the Centre for Adverse Reactions Monitoring (CARM) of tendon disorders in association with quinolone use. Of the 53 reports, 36 cases of tendon disorders were reported following treatment with ciprofloxacin, 16 cases following treatment with norfloxacin and one case following treatment with levofloxacin.
More than a third of reports (36%) were tendon ruptures, with the remainder mostly categorised as tendinitis. Only four patients were reported to be taking steroids together with a quinolone.
The majority of CARM reports from 2007 to mid-2012 described patients aged 60 years and over (83%) with over half (53%) occurring in patients age 75 years and over (Figure 1). There were no reports for patients younger than 30 years of age.
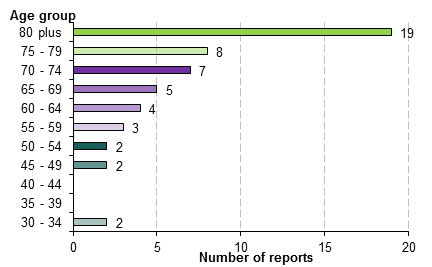
Figure 1: Age of patients in CARM reports of quinolone-associated tendon disorder from 2007 to mid-2012
A review of CARM reports since 2007 identified seven patients who experienced symptoms (tendinitis) in the first 24 hours after receiving a quinolone (Figure 2). In approximately half the cases (54%), the onset of the tendon disorder occurred within one week. In almost all reports (98%), symptoms developed within one month of starting treatment. However, one report described tendon rupture occurring more than six months following the use of a quinolone.
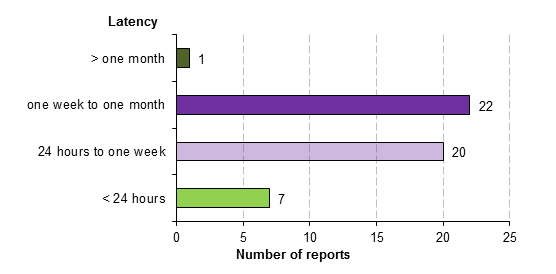
Figure 2: Latency of quinolone-associated tendon disorder reports to CARM from 2007 to mid-2012
Healthcare professionals are encouraged to report these reactions to CARM and to include as much information as possible to help identify other risk factors. More information on these cases can be found by searching SMARS on the Medsafe website.
References
- Khaliq Y, Zhanel GG. 2003. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clinical Infectious Diseases 36: 1404-10.
- van der Linden PD, Sturkenboom MC, Herings RM, et al. 2002. Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ 324: 1306-7.





