Published: 3 March 2016
Publications
Adverse Reaction Reporting in New Zealand – 2015
Prescriber Update 37(1): 2
March 2016
Medsafe and the Centre for Adverse Reactions Monitoring (CARM) would like to thank all those who have submitted reports of suspected adverse reactions over the past year. Submitting reports provides valuable information for the post-marketing monitoring (pharmacovigilance) of medicines and vaccines in New Zealand.
Types of reports
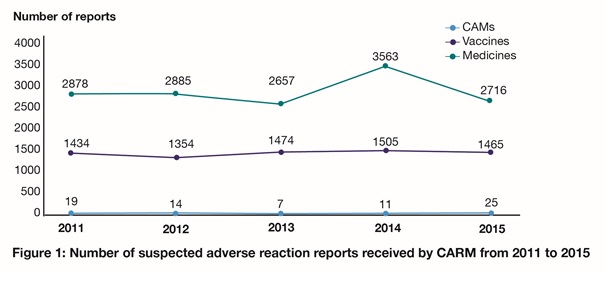
In 2015, CARM received a total of 4206 suspected adverse reaction reports. Of these, 64.6% were associated with medicines, 34.8% were associated with vaccines, and 0.6% were associated with complementary or alternative medicines (CAMs). This is consistent with previous years, with the exception of 2014 where an increase in reports associated with medicines was received (Figure 1).
In 2015, 27% of all reports were considered serious. A serious adverse
reaction is defined, according to internationally agreed criteria, as
any drug-related event that results in death, is life-threatening, requires
or prolongs inpatient hospitalisation, results in persistent or significant
disability or requires intervention to prevent permanent disability,
is a congenital abnormality or is a medically important event. Serious
reports accounted for 39% of the medicine reports, 5% of the vaccine
reports, and 20% of the CAM reports.
Source of reports
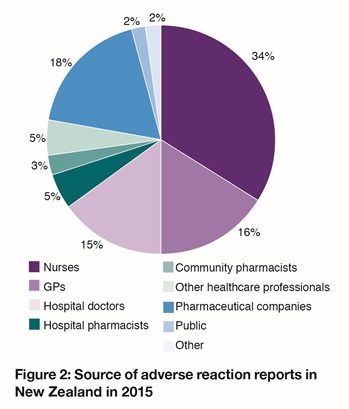
In 2015, nurses continued to be the most frequent reporters of suspected adverse reactions (34% of all reports). Doctors followed closely behind submitting 31% of all reports (GPs reported 16% and hospital doctors reported 15%, Figure 2).
Approximately a quarter of all reports come through GP Practice Management Systems (PMS), which can be used by nurses as well as GPs. These systems use an online reporting form linked to the PMS that automatically pre-populates the patient’s medical history, medicine history and provides an option to include laboratory results. If the report involves a vaccine, details such as batch number and date of administration are included. The report is then sent directly to CARM via a secure electronic pathway.
How to report
Healthcare professionals are encouraged to report any suspected adverse reaction(s) to a medicine, vaccine or CAM to CARM (https://nzphvc.otago.ac.nz/).
Information about how to submit an adverse reaction report can be found on the Medsafe website (www.medsafe.govt.nz/safty/ADR-reporting.asp) or on the CARM website (https://nzphvc.otago.ac.nz/reporting/).
Suspected adverse reactions to medicines, vaccines and CAMs can be reported by:
- completing a freepost yellow card
- phoning the CARM line 0800 4 Monitor (0800-466648)
- downloading a form from either the CARM or Medsafe website
- completing an online report available from the CARM website
- electronic reporting through GP Practice Management Systems
- using the iPhone application (ADR Online).
Remember if in doubt, report it.





