Revised: 21 March 2014
Publications
Adverse Reaction Reporting in New Zealand — 2013
Prescriber Update 35(1): 7–8
6 March 2014
Revised: 21 March 2014
Medsafe and the Centre for Adverse Reactions Monitoring (CARM) would like to thank all those who have submitted reports of suspected adverse reaction and in doing so have contributed to pharmacovigilance in New Zealand.
Reporting of adverse reactions provides valuable information about the use of medicines in clinical practice. Reporting also makes an important contribution to post-market monitoring (pharmacovigilance) in New Zealand.
Before a medicine is approved in New Zealand, safety and efficacy experience is usually limited to its use in clinical trials. However, clinical trials do not always reflect the use of a medicine or vaccine in real life.
In addition, some important reactions are rare and may not be observed until a large number of people have received the medicine or have taken the medicine for a long period. Therefore, it is important to monitor all medicines after they have been approved.
In New Zealand, monitoring of adverse reactions is coordinated by Medsafe and CARM. Medsafe contracts CARM to collect and analyse adverse reaction reports submitted in New Zealand. Medsafe then uses this information to identify possible safety issues.
After further investigation, Medsafe may need to take appropriate action to ensure that the safety of these medicines is improved.
If further information is required to investigate a safety concern, the medicine and safety issue can be placed on Medsafe's scheme. The aim of the scheme is to highlight potential safety concerns to healthcare professionals and encourage reporting.
Information regarding medicines currently being monitored is available on the Medsafe website (www.medsafe.govt.nz/profs/M2MedicinesMonitoring.asp).
If information needs to be communicated about safety concerns, Medsafe uses its early warning system to publish information on its website. Further information about the early warning system, including how to sign up for email alerts, can be found on the Medsafe website (www.medsafe.govt.nz/safety/EWS/EWS.asp).
Adverse Reaction Reports in 2013 — Updated 21 March 2014
In 2013, CARM received a total of 4138 reports of suspected adverse reactions. The number of reports submitted annually in New Zealand has remained consistent over the last five years.
Reports of adverse reactions to medicines make up the majority of the total reports received by CARM (64.2%) during 2013. The remainder of the reports of suspected adverse reactions were associated with vaccines (35.6%) and complementary and alternative medicines (CAMs) (0.2%). The comparative figures for 2012 were 67.8%, 31.8% and 0.3% respectively.
Additional information about suspected adverse reactions reported in New Zealand can be found on the Medsafe website using the Suspected Medicines Adverse Reaction Search (SMARS) (www.medsafe.govt.nz/SMARS/).
Of the reports received, 37% of the medicine reports and only 3% of the vaccine reports were considered serious. For CAMs, 29% of reports described reactions considered as serious.
A serious adverse reaction is determined by the CARM medical assessors according to internationally agreed criteria (ie, resulting in hospitalisation, is life-threatening, fatal, results in disability or requires intervention to prevent permanent disability, or results in a congenital abnormality).
Source of Reports
In 2013, nurses continue to be the healthcare professionals that report the most adverse reactions, followed by GPs and hospital doctors (Figure 1).
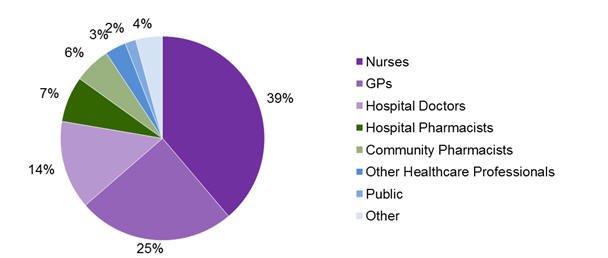
Figure 1: Source of adverse reaction reports from healthcare professionals and consumers in New Zealand in 2013
How to Report
Healthcare professionals and consumers are encouraged to report any suspected adverse reaction to a medicine, vaccine or CAM to CARM.
Information about how to submit an adverse reaction report can be found on the Medsafe website (www.medsafe.govt.nz/safety/report-a-problem.asp) or on the CARM website (https://nzphvc.otago.ac.nz/report/).
Suspected adverse reactions to medicines, vaccines and CAMs can be reported by:
- completing a yellow card
- phoning the CARM line 0800 4 Monitor (0800-466648)
- downloading a form from either the CARM or Medsafe websites
- completing an online report available from either the CARM or Medsafe websites
- electronic reporting through GP software
- using the iPhone application (ADR Online).
Updated 21 March 2014
Adverse reaction report data has been updated since publication. A correction will be included in the printed edition of the June 2014 Prescriber Update.





