Published: March 2013
Publications
Adverse Reaction Reporting in NZ - 2012
Prescriber Update 34(1): 6–7
March 2013
Reporting of adverse reactions provides valuable information about the use of medicines in clinical practice and is an important contribution to pharmacovigilance in New Zealand.
Before a medicine is approved in New Zealand, safety and efficacy experience is usually limited to its use in clinical trials. However, clinical trials do not always reflect the actual use of a medicine or vaccine in real life. In addition, some important reactions are rare and may not be observed until a large number of people have received the medicine or have taken the medicine for a long period. Therefore, it is very important to monitor all the medicines after they have been approved.
In New Zealand, the monitoring of adverse reactions through spontaneous reporting is coordinated by Medsafe and the Centre for Adverse Reactions Monitoring (CARM). CARM is contracted by Medsafe to collect and analyse adverse reactions reports submitted in New Zealand. This information is then provided to Medsafe and the two organisations work together to identify possible safety issues with medicines.
If after further investigation, the safety issue is confirmed, Medsafe takes appropriate action to ensure the safety of these medicines is improved. If further information on potential safety issues is required, the medicine and potential safety issue can be placed on Medsafe's scheme. The aim of is to highlight potential safety concerns identified to healthcare professionals to stimulate further reporting. Information about medicines currently on and the outcome of monitoring can be found on the Medsafe website www.medsafe.govt.nz/profs/M2MedicinesMonitoring.asp
Adverse Reaction Reports in 2012
In 2012, CARM received a total of 4253 suspected adverse reaction reports. The number of reports submitted in New Zealand has remained consistent since 2008.
Adverse reaction reports to medicines made up the majority of the reports received by CARM (67.8%) (Figure 1). The remainder of the suspected adverse reaction reports were associated with vaccines (31.8%) and complementary therapies (CAMs) (0.3%).
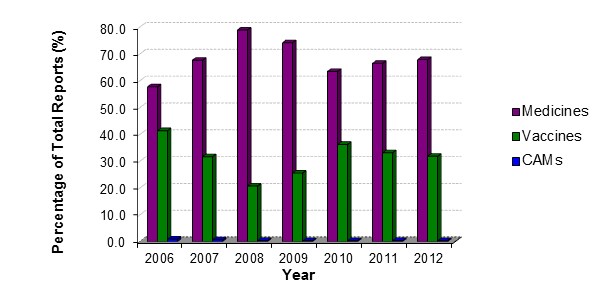
Figure 1: Types of adverse reaction reports received by CARM from 2006 to 2012
Additional information about suspected adverse reactions reported in New Zealand can be found on the Medsafe website using the Suspected Medicines Adverse Reaction Search (SMARS) www.medsafe.govt.nz/SMARS/
Of the medicine and CAM reports received, approximately one third were considered serious. In comparison, only 4.3% of the vaccines reports were considered serious. A serious adverse reaction is determined by CARM according to internationally agreed criteria (ie, resulting in hospitalisation, is life-threatening, fatal, results in a disability or requires intervention to prevent permanent disability, or results in a congenital abnormality).
Source of Reports
In 2012, nurses continued to submit the most adverse reaction reports by healthcare professionals, followed by GPs and hospital doctors (Figure 2).
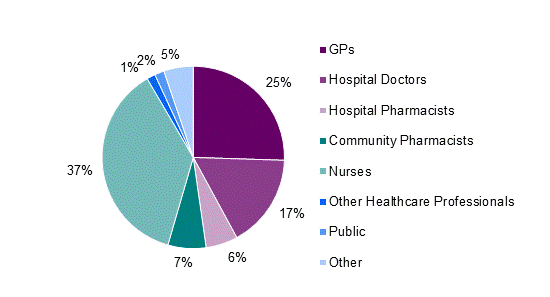
Figure 2: Source of adverse reaction reports from healthcare professionals in New Zealand in 2012
How to Report
Healthcare professionals (and consumers) are encouraged to report any suspected adverse reaction to a medicine, vaccine or CAM to CARM. Suspected adverse reactions to medicines, vaccines and CAMs can be reported by:
- completing a yellow card sent via freepost to CARM
- downloading a form from either the CARM or Medsafe websites
- completing an online report available from either the CARM or Medsafe websites • electronic reporting through GP software
- iPhone app.
Further information about how to submit an adverse reaction report can be found on the Medsafe website www.medsafe.govt.nz/safety/report-a-probelm.asp or on the CARM website https://nzphvc.otago.ac.nz/report/
Medsafe and CARM would like to thank all those who have submitted suspected adverse reaction reports and contributed to pharmacovigilance in New Zealand.





