Published: 1 June 2023
Publications
Reporting anaphylaxis? It’s all in the detail
Published: 1 June 2023
Prescriber Update 44(2): 26–28
June 2023
Report all cases of anaphylaxis to CARM
Anaphylaxis is a life-threatening systemic hypersensitivity reaction that is usually rapid in onset and can be fatal.
Report all suspected or confirmed cases of medicine-induced anaphylaxis to the Centre for Adverse Reactions Monitoring (CARM). CARM can then add an appropriate alert on the national Medical Warning System (MWS) for the patient.
The national Medical Warning System1
The MWS is a national alert service linked to the patient’s National Health Index (NHI) number. It alerts healthcare professionals to known risk factors that may be important when making clinical decisions about a patient. The MWS is particularly important for patients who are unable to communicate, are confused, unconscious or may have forgotten a previous event.
The MWS has 2 types of medicine alerts: Danger and Warning.
- A Danger alert reflects a contraindication to further use for the patient. Used, for example, when the patient has had an anaphylactic-type reaction or any other life-threatening reaction.
- A Warning alert indicates that the medicine has caused the patient significant morbidity and should be avoided.
Some hospitals have systems in place and designated staff who enter Warning alerts into the MWS. Only CARM can place a Danger alert.
Detailed anaphylaxis ADR reports are important
Detailed ADR reports help the medical assessors at CARM determine whether an alert needs to be created in the MWS, and if so, the type of alert.
Medsafe and CARM acknowledge that reporter time and resource constraints may limit the level of clinical detail provided in reports. In addition, some clinical information may not be available at the time of the report submission. As much information as is available at the time of the event is appreciated.
The following details, where available, can support CARM’s causality assessment.
- The time to onset from administering the medicine to the anaphylaxis symptoms.
- Signs and symptoms. The Brighton Collaboration case definition for anaphylaxis can be a useful guide for symptom reporting.
- Tryptase and allergy test results.
Attaching a general practice note, emergency department or hospital summary and specialist clinical letter to the report can provide this useful clinical information.
Submitting follow-up information, as it becomes available, will further assist CARM’s causality assessment. CARM will also update the MWS as appropriate, for example, if allergy testing identifies that the patient is not allergic to the medicine.
Follow-up information can be submitted by emailing carmnz@otago.ac.nz. Please include the report number if available.
Brighton Collaboration case definition for anaphylaxis
The Brighton Collaboration produce case definitions of various conditions including anaphylaxis to help with safety monitoring, primarily of vaccines. These case definitions are an aid to determining diagnostic certainty, with level 1 being the highest certainty and level 5 the lowest.2
The case definition does not determine causality2 and is not a substitute for clinical assessment or a clinical specialist's assessment following formal allergy testing.
CARM use the Brighton Criteria when assessing reports of potential anaphylaxis.
Overview of anaphylaxis reports to CARM
From 1 January 2018 to 30 November 2022, CARM received a total of 720 case reports for 800 medicines suspected of causing immunoglobulin E (IgE)-mediated anaphylaxis. The majority of patients were women (63%), and most patients were reported to have recovered (87%).
The most frequently reported classes of medicines by Anatomical Therapeutic Chemical (ATC) code were systemic antibiotics, muscle relaxants, anti-inflammatories and anti-rheumatic products, and contrast media (Figure 1).
Figure 1: Top 10 classes of medicines, by ATC codes, associated with anaphylaxis in the CARM database, 1 January 2018 to 30 November 2022
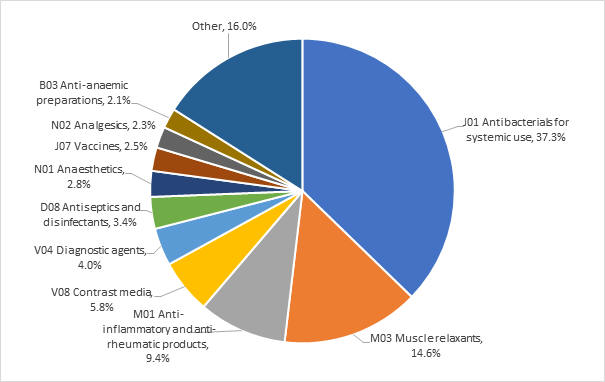
The most frequently reported individual medicines were cefazolin (13.8%), rocuronium (10.1%), amoxicillin with clavulanic acid (7.5%), amoxicillin (3.6%), and diclofenac (3.3%).
For COVID-19 vaccines, there were 128 anaphylaxis reports meeting levels 1–3 of the Brighton Collaboration case definition, up to and including 30 November 2022.
More information
- Prescriber Update June 2020: Alerting the Medical Warning System can save lives!
- The Brighton Collaboration case definition for anaphylaxis
- Report a suspected adverse reaction to CARM
References
- Medsafe. 2020. Alerting the Medical Warning System can save lives! Prescriber Update 42(2): 29-30. URL: medsafe.govt.nz/profs/PUArticles/June2020/Medical-Warning-System.html (accessed 19 April 2023).
- Gold MS, Amarasinghe A, Greenhawt M, et al. 2023. Anaphylaxis: Revision of the Brighton collaboration case definition. Vaccine 41(15): 2605-14. URL: sciencedirect.com/science/article/pii/S0264410X22014256 (accessed 11 April 2023).





