Published: 4 June 2020
Publications
Ondansetron and oral cleft defects
Prescriber Update 41(2): 27–28
June 2020
Ondansetron
Ondansetron is a selective serotonin (5-HT3) receptor antagonist. The approved indications are the management of nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy, and the prevention of post-operative nausea and vomiting. Ondansetron is also used off-label during early pregnancy1.
New Zealand National Collections data show that first trimester use of ondansetron is increasing (Figure 1). The proportion of women who were dispensed ondansetron during the first trimester of pregnancy increased from 0.5 percent in 2009 to 9.6 percent in 2018.
Risk of cleft palate with ondansetron
Two recent epidemiological studies investigated the risk of orofacial cleft defects and other congenital malformations in infants who were exposed to ondansetron in utero, using data from large administrative claims databases in the United States2,3.
The first was a retrospective cohort study of 1,816,414 pregnancies from 2000 to 2013, in which 88,467 (4.9 percent) pregnancies were associated with a prescription for ondansetron in the first trimester. Oral cleft defects occurred in 14.0 per 10,000 exposed infants compared to 11.1 per 10,000 unexposed infants (adjusted relative risk 1.24, 95% CI: 1.03–1.48)2.
The second was a nested case-control study of 864,083 mother-child pairs registered from 2000 to 2014, in which 5,557 mother-child pairs were administered ondansetron in the first trimester. Ondansetron exposure was associated with a numerical increase in oral cleft defects, although this increase did not reach statistical significance (adjusted odds ratio 1.30, 95% CI: 0.75–2.25)3.
The Medicines Adverse Reactions Committee (MARC) discussed the risk of oral cleft defects associated with first-trimester exposure to ondansetron at the March 2020 meeting (see MARC’s remarks in this edition of Prescriber Update). The Committee noted that although the effect sizes in the recently reported studies were small and there is some uncertainty in the data, the current evidence suggests a small increase in the risk of oral cleft defects associated with the use of ondansetron in the first trimester4.
Clinical implications
The recent studies suggest an approximate 25 percent increase in the risk of oral cleft defects with first trimester use of ondansetron, amounting to an additional 3 cases per 10,000 exposed pregnancies.
The data sheets of ondansetron-containing medicines are being updated with information on the increased risk of oral cleft defects associated with first trimester use.
In line with the Code of Health and Disability Services Consumers’ Rights, authorised prescribers must advise the patient when the use of ondansetron in pregnancy is unapproved (off-label) and obtain informed consent5,6.
The possible benefit of using ondansetron during the first trimester of pregnancy must be weighed against the risk of harm. As with all medicines, benefit-risk assessment needs an individualised approach7.
Figure 1: Use of ondansetron during the first trimestera of pregnancy in New Zealand, public hospitalisations data, 2009–2018
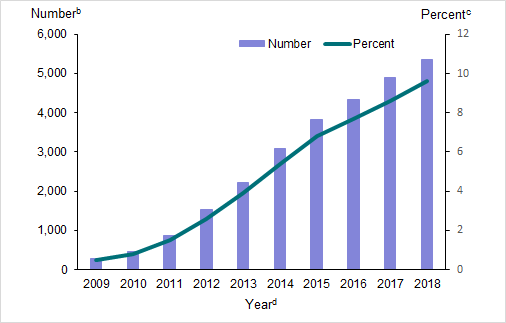
Notes:
- First trimester calculated as the first 13 weeks of pregnancy.
- Number: number of women dispensed ondansetron during the first trimester of pregnancy. Only includes pregnancies that reached at least 20 weeks gestation.
- Percent: number of women dispensed ondansetron/total number of women who reached at least 20 weeks gestation.
- Year: based on the expected delivery date.
Source: National Collections, Ministry of Health. 2020.
References
- Lowe SA, Bowyer L, Beech A, et al. 2019. SOMANZ Guideline for the Management of Nausea and Vomiting in Pregnancy and Hyperemesis Gravidarum. URL: somanz.org/downloads/NVPGUIDELINEFinal.pdf (accessed 11 February 2020).
- Huybrechts KF, Hernández-Díaz S, Straub L, et al. 2018. Association of maternal first-trimester ondansetron use with cardiac malformations and oral clefts in offspring. JAMA 320(23): 2429–37. DOI: 10.1001/jama.2018.18307 (accessed 28 January 2020).
- Zambelli-Weiner A, Via C, Yuen M, et al. 2019. First trimester ondansetron exposure and risk of structural birth defects. Reproductive Toxicology 83(Jan): 14–20. DOI: 10.1016/j.reprotox.2018.10.010 (accessed 28 January 2020).
- Medsafe. 2020. Minutes of the 181st Medicines Adverse Reactions Committee Meeting. URL: medsafe.govt.nz/profs/adverse/Minutes181.htm (accessed 5 May 2020).
- Health & Disability Commissioner. 1996. The HDC Code of Health and Disability Services Consumers' Rights Regulation 1996. URL: hdc.org.nz/your-rights/the-code-and-your-rights/ (accessed 7 April 2020).
- Medsafe. 2014. Use of Unapproved Medicines and Unapproved Use of Medicines 22 October 2014. URL: medsafe.govt.nz/profs/RIss/unapp.asp (accessed 7 April 2020).
- Medsafe. 2019. The Medsafe Files – Episode 12: Benefit-risk (of harm) review. Prescriber Update 40(4): 70–2. URL: medsafe.govt.nz/profs/PUArticles/December2019/The-Medsafe-Files-Episode-12-Benefit-risk-review.htm (accessed 2 April 2020).





