Published: March 2012
Publications
Adverse Reaction Reporting in New Zealand in 2011
Prescriber Update 33(1): 7–9
March 2012
All medicines have risks and benefits.
Before a medicine is released into the New Zealand market, safety and efficacy experience is limited to its use in clinical trials. Some important reactions may not be observed until a large number of people have received the medicine or have taken the medicine for a long period. Continued monitoring of adverse reactions is therefore essential.
National adverse reactions reporting activities (spontaneous reporting) in New Zealand are coordinated by Medsafe and the Centre for Adverse Reactions Monitoring (CARM).
Healthcare professionals (and consumers) can report a suspected adverse reaction to medicines (including vaccines) and complementary therapies (CAMs) by submitting a report through one of the following methods:
- completing a yellow card sent via freepost to CARM
- downloading a form from either the CARM or Medsafe websites
- completing an online report available from either the CARM or Medsafe websites
- electronic reporting through GP software
- Prescriber Update form
- iPhone app.
These reports are subsequently analysed to identify possible safety signals
that require further investigation. Possible safety signals
![]() may appear on the scheme (medsafe.govt.nz/profs/M2MedicinesMonitoring.asp)
and/ or further action may be taken. Information about pharmacovigilance
in New Zealand is available at
www.medsafe.govt.nz/Consumers/ Safety-of-Medicines/Medicines-Safety-andPharmacovigilance.asp.
may appear on the scheme (medsafe.govt.nz/profs/M2MedicinesMonitoring.asp)
and/ or further action may be taken. Information about pharmacovigilance
in New Zealand is available at
www.medsafe.govt.nz/Consumers/ Safety-of-Medicines/Medicines-Safety-andPharmacovigilance.asp.
In 2011, CARM received a total of 4331 suspected adverse reaction reports. This number includes suspected adverse reactions to medicines, vaccines and CAMs. The number of reports submitted has remained consistent for the past four years (Figure 1).
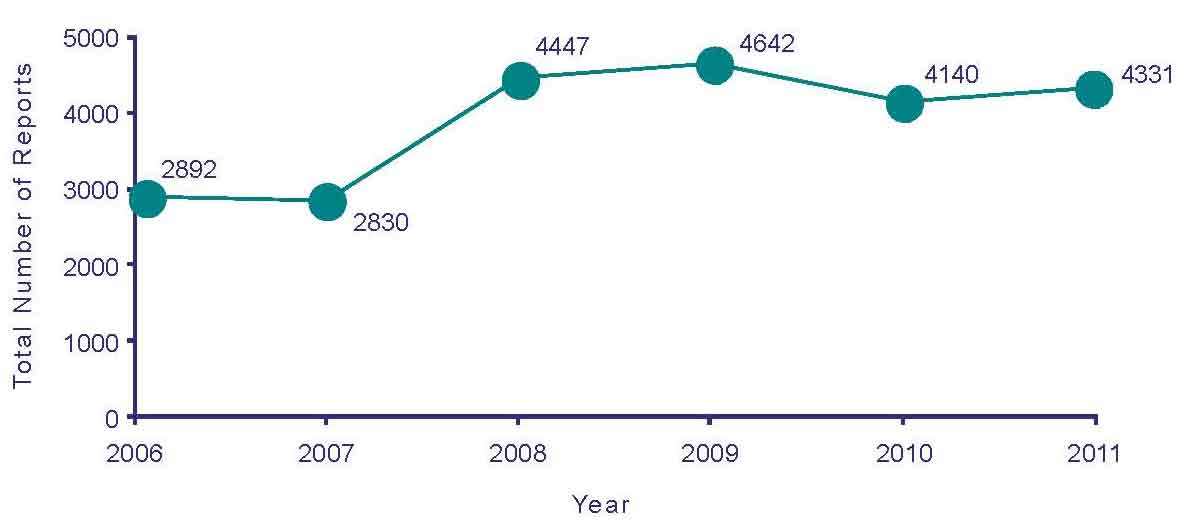
Figure 1: Adverse reaction reports submitted to CARM from 2006 to 2011.
The majority of the reports received by CARM in 2011 were associated with medicines (66.5%) (Figure 2). Vaccines contributed to 33.1% of total suspected adverse reports, with CAMs making up the remaining 0.4%. This is a similar breakdown when compared to the reports received in 2010 (Figure 2).
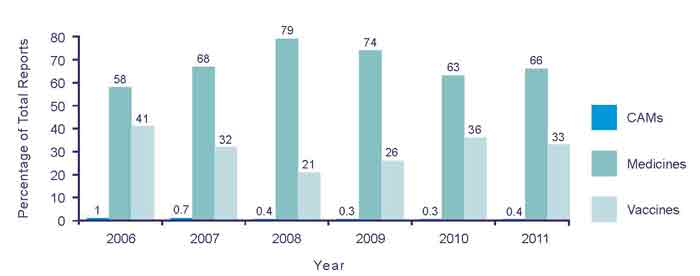
Figure 2: Types of adverse reaction reports from 2006 to 2011.
Reports to CAMs remain low with only 19 submitted in 2011. Possible factors that could explain the low numbers of reports include: adverse reactions to CAMs are rare, adverse reactions to CAMs are not well recognised or a lack of awareness that adverse reactions to CAMs can be reported to CARM.
Examination of the suspected adverse reaction reports received in 2011 showed 21.7% of total reports were considered to be serious. A serious adverse reaction is determined by CARM according to internationally agreed criteria i.e. resulting in hospitalisation, life-threatening, fatal, results in a disability, requires intervention to prevent permanent disability or results in a congenital abnormality.
Approximately one third of reports relating to medicines were considered serious and 10.5% of CAM reports received by CARM were considered serious (Table 1). Interestingly, only 4.3% of vaccine reports submitted were deemed serious.
Table 1: Types of adverse reactions reports for 2011.
| Type | Serious (%) |
Non-Serious (%) |
|---|---|---|
| Medicines | 30.5 | 69.5 |
| Vaccines | 4.3 | 95.7 |
| CAMs | 10.5 | 89.5 |
The source of adverse reaction reports received in 2011 is shown in Figure
3. In 2011, the majority of adverse reaction reports submitted by healthcare
professionals came from nurses, followed by GPs then hospital doctors (Figure
3).
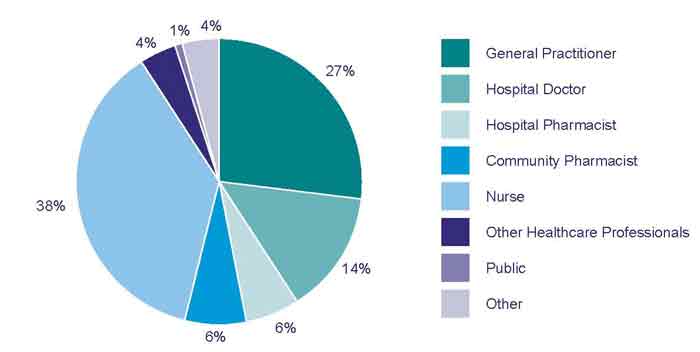
Figure 3: Source of adverse reaction reports from Healthcare Professional in New Zealand in 2011.
Medsafe and CARM would like to thank all those who have contributed to pharmacovigilance in New Zealand by submitting reports of suspected adverse reactions and encourage continued support for 2012.
Adverse reaction reporting provides valuable information about the use of medicines in clinical practice and is a vital part of pharmacovigilance in New Zealand.
Further information about adverse reactions and how to submit an adverse reaction report can be found on the Medsafe website, www.medsafe.govt.nz, or on the CARM website, www.otago.ac.nz/carm.





