Revised: 14 May 2013
Publications
Media Releases for 2009
24 November 2009
Medsafe takes part in global operation targeting online sale of counterfeit medicines
Medsafe has taken part in an international week of action which targeted the online sale of counterfeit and illicit medicines and was coordinated by INTERPOL and the World Health Organization.
Due to an ever-increasing number of websites supplying dangerous and illegal medicines, INTERPOL and the WHO's International Medical Products Anti-Counterfeiting Taskforce coordinated Operation Pangea II involving 25 countries.
During the global operation from 16-20 November 2009, New Zealand Customs detained over 280 packages suspected of containing medicines that entered the country through the International Mail Centre. The detained packages were referred to Medsafe for additional inspection.
“Although the activities last week were part of an international operation, it was business as usual for Customs and Medsafe as intensive detection and inspection are standard practice at the border,” said Mr Derek Fitzgerald, Medsafe’s Manager for Compliance Management.
Mr Fitzgerald said Medsafe’s examination of the packages revealed that a wide range of medicines used to treat a variety of diseases and conditions was being imported.
Although only one of these packages was found to contain a counterfeit product (Cialis, which is used to treat erectile dysfunction), other products of concern to Medsafe were found. These products included performance- and image-enhancing drugs (typically, anabolic steroids), products purporting to be herbal in nature that have been found in the past to contain western medicines, and items without any labelling or packaging that would allow the products to be correctly identified.
Mr Fitzgerald warned the public about the dangers of purchasing medicines on the Internet. "Medicines that had not been evaluated by Medsafe for their quality, safety and efficacy through the New Zealand assessment process could put the health of consumers at risk. Our experience is that medicines purchased on the Internet may be of poor quality, adulterated, counterfeit or manufactured in countries where regulation and compliance with accepted standards is poor," he cited.
Many of the packages examined were found to contain prescription medicines that can only be obtained in New Zealand following a consultation with an authorised prescriber (usually, a medical practitioner).
Mr Fitzgerald also advised that all intended recipients of packages containing prescription medicines would be contacted by Medsafe to ensure they were legally entitled to receive the products.
Countries involved in Operation Pangea II were: Australia, Austria, Belgium, Canada, Czech Republic, Denmark, France, Germany, Ireland, Israel, Italy, Liechtenstein, Netherlands, New Zealand, Norway, Portugal, Singapore, South Africa, Spain, Sweden, Switzerland, Thailand, the United Kingdom and the United States.
ENDS
23 November 2009
Medsafe Warns of 65 Overseas Websites offering Unapproved Herbal Products
Medsafe today warned the public of 65 overseas websites that purport to be based in New Zealand and are touting unapproved herbal products.
"We would like to warn the public that these websites are selling products that have not been assessed for quality, safety and efficacy in New Zealand. Neither has consent been given to the advertising or distribution of these products in New Zealand," said Mr Derek Fitzgerald, Medsafe's Manager for Compliance Management.
Following complaints from consumers here and overseas, Medsafe's Investigation and Enforcement team began investigations in August into an Internet-based business operation known as "Gordon's Herbal Research Centre" (GHRC).
The company operates at least 65 websites offering "cure alls" for various illnesses and medical conditions. It claims that the herbal products had undergone clinical trials conducted "under the regulations of FDA and the New Zealand Health Authority". The websites also provide details of a New Zealand-based postal box, giving the impression that the company is based here.
"Our investigations indicate that all 65 websites are operated from overseas and are a money-making scam," according to Mr Fitzgerald.
"Contrary to the information on these websites, this operation has no physical presence in New Zealand but is run by an individual based in Pakistan."
Mr Fitzgerald cited that one of the products promoted on these websites, Reneton, purportedly can successfully treat polycystic kidney disease, a condition for which only symptomatic relief is available.
He also advised consumers "not to purchase medicines over the Internet because it is very difficult to determine whether the products offered are effective, safe, and legally supplied. The quality of medicines purchased over the Internet cannot be guaranteed and consumers may be exposed to poor quality, counterfeit products that carry serious health risks."
ENDS
Background
- Medsafe and NZ Customs took part in an international operation from 16-20 November 2009 targeting the online sale of counterfeit and illicit medicines.The International Week of Internet Action was coordinated by INTERPOL and the World Health Organization's (WHO) International Medical Products Anti-Counterfeiting Taskforce (IMPACT) to highlight the dangers of buying medicines online, and was supported by the Permanent Forum on International Pharmaceutical Crime.
- The Permanent Forum on International Pharmaceutical Crime (PFIPC) is an international forum formed in 1998 that aims to encourage inter-agency cooperation in combating the serious threat to public health from the various forms of pharmaceutical crime. The PFIPC works in cooperation with a separate Forensic Group, which provides scientific expertise, and with other interested bodies including the World Health Organization, the World Customs Organization, and INTERPOL. New Zealand is a member of the PFIPC.
- Medsafe has so far identified at least 65 product websites operated
by Gordon's Herbal Research Centre:
- www.barbeton.com
- www.betneton.com
- www.breneton.com
- www.celceton.com
- www.celdeton.com
- www.celseton.com
- www.cesteton.com
- www.chondreton.com
- www.cloreton.com
- www.cremeton.com
- www.creseton.com
- www.delecreton.com
- www.dermeton.com
- www.epidmenton.com
- www.felceton.com
- www.flameton.com
- www.flemeton.com
- www.gabeton.com
- www.gesteton.com
- www.glenteton.com
- www.graveton.com
- www.greneton.com
- www.greveton.com
- www.hysmeton.com
- www.kemeton.com
- www.kleneton.com
- www.larneton.com
- www.lemeton.com
- www.lichreton.com
- www.luseton.com
- www.melseton.com
- www.meyseton.com
- www.nedeton.com
- www.neteton.com
- www.neureton.com
- www.olceton.com
- www.osmeton.com
- www.osteton.com
- www.palmeton.com
- www.pebneton.com
- www.pempheton.com
- www.perteton.com
- www.peyreton.com
- www.phlereton.com
- www.phrenaton.com
- www.phreneton.com
- www.pigmeton.com
- www.polpeton.com
- www.preleton.com
- www.prigmeton.com
- www.proleton.com
- www.reneton.com
- www.repeton.com
- www.rheneton.com
- www.rheumeton.com
- www.sebeton.com
- www.sedeton.com
- www.sepreton.com
- www.trendeton.com
- www.treneton.com
- www.troneton.com
- www.urethreton.com
- www.uvieton.com
- www.vegeton.com
- www.veraceton.com
7 October 2009
Cough and Cold Medicines Should Not be Given to Children Under Six
Medsafe has accepted the recommendation of the Cough and Cold Review Group that cough and cold medicines should not be given to children under six years of age in light of limited evidence that these medicines work in children and reports of side effects.
Early this year, Medsafe convened the Cough and Cold Review Group to carry out a thorough review of the benefits and risks of using cough and cold medicines in children following advice by Canadian and UK authorities against the use of these products for children under six. The Cough and Cold Review Group consisted of pharmacists, doctors, paediatricians and representatives from the New Zealand Centre for Adverse Reactions Monitoring, the Medicines Adverse Reactions Committee, Plunket, the pharmaceutical industry and the public.
"Medsafe will work closely with manufacturers to implement the Cough and Cold Review Group's recommendations as soon as possible," Medsafe Group Manager Dr Stewart Jessamine said.
"More information will be provided to healthcare professionals and consumers as the recommendations are implemented," he added.
Further information on the recommendations made by the Cough and Cold
Review Group, including the minutes of the Group’s meetings, can be found
on the Medsafe website:
(www.medsafe.govt.nz/hot/alerts/CoughandCold/CoughandCold.asp)*
"After full consideration of the data, the Group has recommended that oral cough and cold medicines should not be used in children under six with the exception of those containing only bromhexine," Cough and Cold Review Group Chair Andi Shirtcliffe said.
"Our review found that the balance of risks and benefits for the use of these medicines in children under six is unfavourable," Mrs Shirtcliffe cited.
As with all medicines, consumers and healthcare professionals are encouraged to report all suspected adverse reactions to the Centre for Adverse Reactions Monitoring (CARM).
ENDS
* Please note the information contained in this link has been superseded (the recommendations remain the same). The following link contains the most up-to-date information: www.medsafe.govt.nz/hot/alerts/CoughandCold/InfoOct2009.asp
Background:
- Medsafe is the New Zealand Medicines and Medical Devices Safety Authority, and is part of the Ministry of Health.
- The Cough and Cold Review Group considered that the use of oral medicines containing bromhexine only or intra-nasal decongestants (such as oxymetazoline and xylometazoline) should remain restricted to adults and children two years of age and over.
- More information on reporting adverse reactions to medicines can be found at: https://pophealth.my.site.com/carmreportnz/s/.
24 September 2009
No conclusive evidence linking insulin glargine to higher cancer risk
Medicines regulatory authorities in New Zealand have found no conclusive evidence that the use of insulin glargine is associated with increased risk of developing cancer, saying findings of recent studies may be due to chance.
Insulin glargine (brand name: Lantus) is used once daily for control of diabetes, often in combination with a short acting sulfonylurea or other insulin.
Medsafe and the Medicines Adverse Reactions Committee (MARC) have reviewed the safety of insulin glargine following the publication of four studies which suggested that this insulin may be associated with a higher risk of cancer.
"The MARC concluded that there were significant problems with these studies and could not determine whether there was an increased risk of cancer associated with taking glargine. It is possible the reported findings arose by chance," said Dr Marius Rademaker, acting chair of MARC.
"The MARC also noted that people with diabetes seem to have a somewhat higher risk of cancer than people without diabetes. This risk may be reduced by appropriate lifestyle changes," according to Dr Rademaker.
"As a precaution, we advise that until further information becomes available, patients should only use insulin glargine when it has specific benefits for them. Patients currently on glargine are encouraged to discuss this with their GP or specialist and are advised not to change their treatment without medical advice."
Medsafe Group Manager Dr Stewart Jessamine said patients should report all suspected adverse reactions to the Centre for Adverse Reactions Monitoring (CARM).
ENDS
Background information:
- MARC advises the Minister of Health on issues relating to safety of medicines. The minutes of the MARC's 10 September 2009 meeting will be published on the Medsafe website.
- Medsafe is the New Zealand Medicines and Medical Devices Safety Authority, and is part of the Ministry of Health.
- More information on reporting adverse reactions to medicines can be found on: https://pophealth.my.site.com/carmreportnz/s/.
For queries, please contact: Luz Baguioro, Media Advisor (04 496 2349, 021 802 622)
17 September 2009
JOINT MEDIA RELEASE – MINISTRY OF HEALTH AND PHARMAC
IMPORT OF APOTEX PRODUCTS UNDER CLOSE MONITORING
This is an update to our release on Monday 14 September.
The Ministry of Health’s drug regulatory arm Medsafe has negotiated an agreement with pharmaceutical manufacturer Apotex to temporarily restrict importation of medicines produced by Apotex at its two manufacturing sites in Toronto, Canada.
This follows a similar move in the United States last week, where further imports of Apotex medicines manufactured from these two sites has ceased. Apotex has also voluntarily recalled 40 medicines from the US market and three from the Canadian market. No batches of the medicines recalled in the US and Canada are available in New Zealand.
The agreement to temporarily restrict imports in New Zealand is effective today. It has come about after concerns were identified by the US Food and Drug Administration (FDA) when they found deviations from accepted manufacturing processes at two Apotex manufacturing plants in Toronto, Canada.
Medsafe Group Manager Dr Stewart Jessamine says the temporary restriction on imports is a precautionary measure which will remain in place until Medsafe is satisfied that Apotex had improved its manufacturing practices. "At this point, there is no reason for people to be concerned about taking any medicines manufactured by Apotex. There is no suggestion that people have been put at risk by taking any medicines made at any Apotex plants," Dr Jessamine said. “The issue that has been identified relates to the assurance of quality manufacture at the Canadian plants.”
Apotex makes and markets a wide range of medicines which are supplied to 115 countries worldwide. Medsafe has been working closely with PHARMAC, New Zealand's pharmaceutical funding and purchasing agency, to identify which Apotex medicines are available in New Zealand. PHARMAC funds 30 medicines supplied by Apotex, of which 20 are made at the two sites.
PHARMAC medical director, Peter Moodie, said Apotex products are widely used in New Zealand as they are in many countries and PHARMAC was keen to ensure continued supply of high quality medicines for New Zealanders.
"As a precaution, PHARMAC has begun discussions with other companies to ensure that future supply requirements can be met in the event that the import restriction is required for longer than expected. Our first priority is to ensure New Zealanders can have ongoing access to their medicines," Dr Moodie said
Since the cessation of imports to the US last week, Medsafe has been in discussions with the FDA, Health Canada, PHARMAC and Apotex to obtain up to date, accurate information about the international situation. Health Canada is currently auditing the two affected Apotex sites in Etobicoke and Weston. Due to the size and scale of Apotex's operations in Toronto, the site visits alone will take at least a month. The audit is being carefully followed by medicine regulatory authorities around the world.
"We will continue to actively monitor this situation and will inform healthcare professionals and consumers if the situation changes significantly", he said.
For more information www.health.govt.nz/publications
or contact:
Simon England
Communications Manager
PHARMAC
Phone 021 863 342
or Karalyn van Deursen
Senior Media Advisor
Ministry of Health
Phone 021 832 459
ENDS
Questions and answers
What is the issue?
Medsafe was notified by Apotex on 7 September 2009 of concerns with two manufacturing plants owned by Apotex. The Apotex notification follows an FDA audit that found deviations from accepted manufacturing processes at two of Apotex’s manufacturing plants based in Toronto (Weston, Etobicoke).
The FDA’s concerns relate to deficiencies in quality assurance processes and in documentation relating to the manufacture of medicines. As a result the FDA stopped the import of product manufactured at the two sites into the US.
Who are Apotex?
Apotex is a Canadian-based company that makes and markets medicines. Apotex supplies medicines to 115 countries including New Zealand, Australia, Canada, US, and the UK. It has sales of over Canadian $1 billion annually.
What action is Medsafe taking?
Medsafe is working closely with other regulatory authorities and Apotex to obtain assurance that issues identified in the FDA audit have been resolved by Apotex.
In the interim, Apotex has agreed to import restrictions of medicines manufactured from the two affected sites into New Zealand.
Medsafe is working closely with Apotex and PHARMAC to ensure the import restrictions do not result in any medicine supply shortages.
What action are other countries taking?
Apotex has ceased the further export of medicines manufactured from both of the affected plants into the United States. Apotex has also voluntarily recalled 40 batches of medicines from the US market.
In Canada, Health Canada is conducting an audit of the sites concerned. The sites continue to supply medicines to Canada. Apotex has voluntarily recalled three batches of medicines.
Information provided to Medsafe indicates that no batches of medicines voluntarily recalled in US or Canada are available in New Zealand.
Medsafe remains in regular contact with international regulatory authorities to monitor any action being taken overseas.
What Apotex products made at either of the two affected sites are available in New Zealand?
See the table below in Appendix 1 for the list of products manufactured by the two affected sites that are funded by PHARMAC or available over the counter in New Zealand.
Advice for Patients
Should I stop taking my medicine if it is made by Apotex?
No. There is no indication that patients have experienced problems from using Apotex medicines. In addition, the batches of medicines voluntarily recalled in the US and Canada are not available in New Zealand.
It is important that you do not stop taking your medicine as abruptly stopping some medicines can be dangerous.
Do I need to see my doctor?
No, you should keep taking your medication as prescribed. Medsafe and PHARMAC will be communicating with healthcare professionals to keep them informed of further developments as necessary. If you are concerned talk to your doctor next time you visit.
If the situation changes, Medsafe and PHARMAC will make a public announcement.
Who can I contact for further information?
You should contact a healthcare professional such as your GP or pharmacist. For general information about the medicines affected check the Ministry of Health website http://www.health.govt.nz or call Healthline on 0800 611 116. Healthline is open 24 hours a day seven days a week.
Appendix 1 – List of products manufactured by the two affected sites that are funded by PHARMAC or available over the counter in New Zealand
A list of all medicines manufactured at the two affected sites that have consent for distribution in New Zealand is available on the Medsafe website.
- Medicines funded
Product Use Allopurinol Gout Amlodipine Blood pressure Bromocriptine Parkinson’s disease Captopril Hypertension Cimetidine Heartburn and peptic ulcers Clopidigrel Blood clots Diclofenac Inflammation and pain Doxazosin Blood pressure Gliclazide Type 2 diabetes Moclobemide Depression Nadolol Blood pressure and migranes Oxybutynin Urinary and bladder difficulties Prazosin Blood pressure Prednisone Allergic disorders, ulcerative colitis, psoriasis and arthritis Primidone Epilepsy Selegiline Parkinson’s disease Terazosin Blood pressure Terbinafine Skin infections Timolol maleate Blood pressure and glaucoma Zopiclone Insomnia - Over-the-counter medicines
Product Use Apo-Diclo Pain and inflammation Apo-Cetirizine Allergies/hayfever Apo-Famotidine Heartburn and peptic ulcers Apo-Ibuprofen Pain and inflammation Ibucare (ibuprofen) Pain and inflammation Apo-Loperamine Diarrhoea Apo-Loratadine Allergies/hay fever Apo-Ranitidine Heartburn and peptic ulcers
17 September 2009
Full list of products
The following table provides a list of products manufactured by the two affected Apotex sites that may be available in New Zealand.
This table includes products funded by PHARMAC and those not currently funded by PHARMAC.
- Prescription Medicines
Brand (Generic Name) Products Common Use Apo-Acyclovir Tablet 200 mg
Tablet 400 mg
Tablet 800 mgAnti-viral medicine Apo-Alendronate Tablet 70 mg Osteoporosis Apo-Allopurinol Tablet 100 mg
Tablet 300 mgGout Apo-Amlodipine Tablet 5 mg
Tablet 10 mgBlood pressure Apo-Atenolol Tablet 50 mg
Tablet 100 mgBlood pressure Global Atenolol Tablet 50 mg
Tablet 100 mgBlood pressure Apo-Baclofen Tablet 10 mg
Tablet 20 mgMuscle relaxant Alpha-Baclofen Tablet 10 mg
Tablet 20 mgMuscle relaxant Apo-Bicalutamide Tablet 50 mg
Tablet 100 mgProstate cancer Apo-Bisacodyl Enteric Coated Tablet 5 mg Laxative Apo-Bromocriptine Tablet 2.5 mg
Tablet 5 mgParkinson’s disease Apo-Cal (Calcium carbonate) Film Coated Tablet 250 mg
Film Coated Tablet 500 mgCalcium supplement Apo-Captopril Tablet 12.5 mg
Tablet 25 mg
Tablet 50 mg
Tablet 100 mgBlood pressure Apo-Cilazapril/HCTZ Film Coated Tablet 5 mg/12.5 mg Blood pressure Apo-Cimetidine Film Coated Tablet 200 mg
Film Coated Tablet 400 mg
Film Coated Tablet 800 mgHeartburn and peptic ulcers Apo-Citalopram Film Coated Tablet 20 mg Depression Apo-Clomipramine Film Coated Tablet 25 mg Depression Apo-Clopidogrel Tablet 75 mg Blood clots Ferriprox Film Coated Tablet 500 mg Reduces iron Apo-Diclo Enteric Coated Tablet 25 mg
Enteric Coated Tablet 50 mg
SR Modified Tablet 75 mg
SR Modified Tablet 100 mgPain and inflammation Apo-Diltiazem CD Modified release capsule 120 mg
CD Modified release capsule 180mg
CD Modified release capsule 240 mg
CD Modified release capsule 300 mgBlood pressure Apo-Doxazosin Tablet 1mg
Tablet 2 mg
Tablet 4 mgBlood pressure Apo-Famotidine Film Coated Tablet 20 mg
Film Coated Tablet 40 mgHeartburn and peptic ulcers Apo-Fluoxetine Capsule 10 mg
Capsule 20 mgDepression Apo-Gabapentin Film Coated Tablet 600 mg
Film Coated Tablet 800 mg
Capsule 100 mg
Capsule 300 mg
Capsule 400 mgNerve pain and epilepsy Apo-Gemfibrozil Film Coated Tablet 600 mg
Capsule 300 mgHigh cholesterol Apo-Glibenclamide Tablet 2.5 mg
Tablet 5 mgType 2 diabetes Apo-Gliclazide Tablet 80 mg Type 2 diabetes Apo-Ibuprofen Film Coated Tablet 200 mg Pain and inflammation Apo-Levocarb Tablet 25mg/100 mg
Tablet 25 mg/250 mgParkinson’s disease Apo-Loperamide Tablet 2mg Diarrhoea Apo-Mefenamic Acid Capsule 250 mg Pain and inflammation Apo-Megestrol Tablet 160 mg
Tablet 40 mgCancer Apo-Metformin Tablet 500 mg
Tablet 850 mgType 2 diabetes Metformin tablets Tablet 500 mg
Tablet 850 mgType 2 diabetes Apo-Moclobemide Film Coated Tablet 150 mg
Film Coated Tablet 300 mgDepression Apo-Nadolol Tablet 40 mg
Tablet 80 mgBlood pressure Apo-Naproxen SR Modified Release Tablet 750 mg Pain and inflammation Apo-Oxybutinin Tablet 5mg Urinary and bladder difficulties Apo-Paroxetine Film Coated Tablet 20 mg Depression Apo-Pindolol Tablet 5 mg
Tablet 10 mg
Tablet 15 mgBlood pressure Apo-Pravastatin Tablet 10 mg
Tablet 20 mg
Tablet 40 mgHigh cholesterol Apo-Prazo (Prazosin) Tablet 1 mg
Tablet 2 mg
Tablet 5 mgBlood pressure Apo-Prednisone Tablet 1 mg
Tablet 2.5 mg
Tablet 5 mg
Tablet 20 mgAllergic disorders, ulcerative colitis, psoriasis and arthritis Apo-Primidone Tablet 250 mg Epilepsy Apo-Rantidine Film Coated Tablet 150 mg
Film Coated Tablet 300 mgHeartburn and peptic ulcers Apo-Risperidone Film Coated Tablet 0.5 mg
Film Coated Tablet 1 mg
Film Coated Tablet 2 mg
Film Coated Tablet 3 mg
Film Coated Tablet 4 mgAnti-psychotic Apo-Selegiline Tablet 5 mg Parkinson’s disease Apo-Sertraline Tablet 50 mg
Tablet 100 mgDepression Apo-Sotalol Tablet 80 mg
Tablet 160 mgBlood pressure Apo-Sulindac Tablet 200 mg Pain and inflammation Apo-Terazosin Tablet 1 mg
Tablet 2 mg
Tablet 5 mgBlood pressure Apo-Terbinafine Tablet 250 mg Skin infections (fungal) Apo-Tiaprofenic Acid Tablet 200 mg
Tablet 300 mgPain and inflammation Apo-Timol (Timolol) Tablet 10 mg Blood pressure and glaucoma Apo-Topiramate Tablet 25 mg
Tablet 50 mg
Tablet 100 mg
Tablet 200 mgEpilepsy Apo-Zopiclone Film Coated Tablet 7.5 mg Insomnia - Over-the-counter medicines
Medicine Products Common use Apo-Diclo Enteric Coated Tablet 25 mg Pain and inflammation Apo-Cetirizine Tablet 10mg Allergies/hayfever Apo-Famotidine Film Coated Tablet 20 mg Heartburn and peptic ulcers Apo-Ibuprofen Film Coated Tablet 200 mg Pain and inflammation Ibucare (ibuprofen) Film Coated Tablet 200 mg Pain and inflammation Apo-Loperamine Tablet 2 mg Diarrhoea Apo-Loratadine Tablet 10 mg Allergies/hay fever Apo-Ranitidine Film Coated Tablet 150mg Heartburn and peptic ulcers
14 September 2009
Medsafe closely monitoring Apotex
The Ministry of Health’s drug regulatory arm Medsafe is closely monitoring Apotex, Canada’s largest medicines manufacturer, following the United States placing an import ban on medicines produced at two manufacturing sites by this company.
The United States ban was put in place after the Food and Drug Administration (FDA) found deviations from accepted manufacturing processes at two Apotex manufacturing plants in Toronto in Canada.
In response to the FDA findings, Apotex has voluntarily recalled 40 medicines for the US market and three from the Canadian market. None of the affected batches of recalled medicines are available in New Zealand.
Medsafe Group Manager Dr Stewart Jessamine says its Canadian regulatory equivalent Health Canada started an audit this week of the two Canadian sites. The audit is being carefully followed by medicine regulatory authorities around the world.
Since last week's US import ban, Medsafe has been working with the FDA, Health Canada, PHARMAC and Apotex to determine the implications for New Zealand products manufactured at the affected sites.
"Like many other countries, Apotex products are used in New Zealand and both agencies are working closely on the issue to provide assurance that the public has access to affordable high quality medicines," says PHARMAC Medical Director Peter Moodie.
"Although the FDA report has raised questions about the manufacturing process at these two Canadian plants, there is no evidence that this has led to an increased risk to patient welfare for medicines made at these plants," Dr Jessamine said. "Our advice to patients at this time is to keep taking their medicines as prescribed."
Dr Jessamine says: "While the Health Canada audit will provide us with further information about these manufacturing sites, given the size and complexity of the manufacturing sites the site visits themselves are likely to take up to a month.
"We are actively managing this emerging situation and will inform healthcare professionals and consumers if the situation changes significantly", he said.
ENDS
For further information please contact:
Karalyn van Deursen
Senior Advisor – Media Relations
Ministry of Health
Phone (04) 496 2115 or (021) 832 459
4 September 2009
Medsafe reviews dextropropoxyphene-containing medicines: Capadex and Paradex
Medsafe has started a statutory review of the safety and efficacy of all medicines containing dextropropoxyphene, Medsafe's Group Manager Dr Stewart Jessamine said.
Medicines containing dextropropoxyphene are analgesics used to treat chronic moderate pain. Two medicines containing dextropropoxyphene are currently approved for use in New Zealand. The brand names of these products are Capadex and Paradex. Further information about these medicines is available on their data sheets on the Medsafe website (www.medsafe.govt.nz).
"Should this review show that the balance of risks and benefits is unfavourable, this may lead to the withdrawal of these medicines in New Zealand. At the earliest, a final decision on the outcome will not be available until the last quarter of the year," Dr Jessamine said.
Medsafe initially reviewed the safety of these medicines in 2005. There were concerns around the risk of fatal heart problems, depression of breathing and coma especially in overdose. It was noted that some overdoses had occurred accidentally. Following consultation with healthcare professionals, Medsafe concluded that there was a clinical need for these products for some patients.
In late 2005, Medsafe introduced prescribing restrictions to limit use of these medicines to those patients most likely to benefit from their use. In addition, Medsafe required the manufacturers of these medicines to conduct a drug utilisation study to determine if the restrictions had produced changes in prescribing behaviour.
Dr Jessamine said the results of the study investigating the use of these medicines was reviewed by the Medicines Adverse Reactions Committee (MARC) in June this year.
“The study showed that over half the prescriptions issued for Paradex were not in accordance with the prescribing restrictions. In addition, a significant number of prescriptions were issued for patients, including children, and the treatment of conditions outside of those assessed and approved by Medsafe.”
The MARC concluded that the restrictions on the use of Capadex and Paradex have not changed prescribing habits and recommended to Medsafe that it review the benefits and risks of the continued availability of these medicines.
ENDS
For queries, please contact: Luz Baguioro, Media Advisor (04 496 2349, 021 802 622)
Additional information
The Medicines Adverse Reactions Committee (MARC) advises the Minister of Health on medicines safety issues.
- Details of the 2005 review of dextropropoxyphene containing products
can be found in the Minutes of the MARC:
(www.medsafe.govt.nz/profs/adverse/Minutes138.htm#3.2)
(www.medsafe.govt.nz/profs/adverse/minutes122.htm#2.2.7) - Medsafe is the New Zealand medicines and medical devices safety authority, and is a business unit of the Ministry of Health.
- A statutory review of the risks and benefits of a medicine is mandated by Section 36 of the Medicines Act 1981. Section 36 of the Act gives the Director-General of Health the power to require a medicine sponsor to supply information to support the efficacy and safety of that medicine. If a review of this data is unfavourable, the Minister of Health may then prohibit the supply of the medicine.
- The data sheet is a summary of the known effects of a medicine, and includes information on the approved uses of the medicine (indications). Data sheets are published on the Medsafe website.
- The approved indications of Paradex and Capadex are "for the relief of chronic pain of moderate severity. These medicines should only be prescribed to patients in whom treatment of therapeutic doses of alternative therapeutic groups, including combination products, have been used and found to lack analgesic efficacy or have unacceptable adverse effects in the individual patient."
- Paradex is not recommended for administration to children.
- The administration of Capadex is not recommended for children under 14 years.
- Medsafe is aware that the European Medicines Agency (EMEA) has completed a review of the safety and effectiveness of dextropropoxyphene-containing medicines and concluded that the benefits of dextropropoxyphene do not outweigh its risks. Therefore, the EMEA is recommending that all dextropropoxyphene-containing products are withdrawn in Europe. The timing of product withdrawal will be decided by the medicines regulatory agency of each country.
- Medsafe is aware that the US Food and Drug Administration is taking action to improve the safe use of medicines containing propoxyphene (dextropropoxyphene). The FDA has not recommended that these medicines are withdrawn
7 May 2009
Update on the use of "teething" gels containing choline salicylate in children – results of Medsafe's review
Please attribute to Dr Stewart Jessamine, Group Manager, Medsafe
In New Zealand two products contain choline salicylate, Bonjela Teething Gel and Bonjela Mouth Ulcer Gel.
Medsafe has now reviewed the available data and is satisfied that the safety of these products is acceptable when used at the recommended dose. These products have been used in New Zealand for over 30 years and to date there have been no reports of adverse reactions received by the Centre for Adverse Reactions Monitoring (CARM).
Data obtained from the New Zealand Poisons Centre has however highlighted that these products are sometimes used too much and too often, and have been involved in a number of unintentional overdoses. Medsafe therefore takes this opportunity to remind parents and carers about the importance of reading the information provided with the medicine and adhering to the recommended dose. Parents and carers are also reminded to keep all medicines out of sight and reach of children.
Parents and carers with any questions about the use of these medicines in children are recommended to talk to a healthcare professional.
Notes to Editor
- Medsafe has reviewed the data considered by the Medicines and Healthcare products Regulatory Agency (MHRA) that led to the decision to contraindicate the use of topical oral choline salicylate gels in children under 16 years of age.
- The MHRA review was based on a case published in the British Medical Journal in June 2008. The article described a suspected case of Reye’s syndrome in a 20 month old child following the use of Bonjela oral gel. The MHRA concluded that the symptoms described in this case could not be considered as Reye’s syndrome and were more likely due to salicylate toxicity from overuse of the product. The MHRA decided to contraindicate the use of these products in children under 16 years of age due to a theoretical risk of Reye’s syndrome.
- Medsafe's advice follows a review of the MHRA decision, data obtained from the manufacturer of Bonjela, and data obtained from CARM and the New Zealand Poisons Centre. Medsafe's advice is consistent with that of the Irish Medicines Board (IMB). As far as Medsafe is aware, the IMB is the only regulatory agency to release advice in response to the MHRA's decision to date.
- The New Zealand Poisons Centre have advised Medsafe that they have received 279 calls relating to the use of these products in children since 2002.
- Reye's syndrome is a very rare condition that causes serious liver and brain damage. Almost all recorded cases of Reye's syndrome have occurred in children. The cause of Reye's syndrome is unknown, but there is evidence to suggest that two factors may contribute towards children developing the condition; previous viral infection and exposure to aspirin.
- Please note that there is no evidence that links Reye's syndrome to the use of choline salicylate containing products such as Bonjela.
13 March 2009
Health warning issued under Section 98 of the Medicines Act 1981 - Samoan and Tongan medicines
The Director-General of Health, Stephen McKernan, is warning the public about the potential health dangers associated with four unapproved medicines manufactured in Samoa and Tonga and sold from general retailers in New Zealand.
This statement about the four medicines is issued by the Director-General under Section 98 of the Medicines Act 1981 and follows a statement made on 11 December 2008 in relation to three similar Samoan products.
Three of the medicines being distributed are labelled as originating from Multipharm in Apia, Samoa. It is believed that they have been imported by a number of individuals and are sold by various retail outlets. The fourth product appears to be manufactured in Tonga and the extent of distribution is not yet known. The products are:
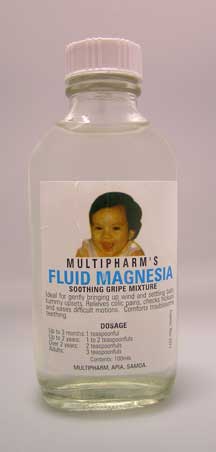
'Multipharm's Fluid Magnesia', a product that is labelled as being suitable for babies younger than 3 months to be used for bringing up wind, settling tummy upsets and relieving colic pains The label does not state the ingredients that are included in the product.
'Multipharm Vai Lafa', a product intended for the treatment of ringworm. The label states that the product contains salicylic acid.
'Multipharm's Vai Tane', a product intended for the treatment of fungal infections. The label states that the product contains salicylic acid.
Vaikahi Mixture (Tukia Pharmaceuticals Ltd) is labelled for uses including bloating and constipation. The label states the product contains magnesium sulphate and light magnesium carbonate.
The safety, quality and efficacy of these medicines are unknown as they have not been assessed and approved for supply in New Zealand through the medicines approval process.
In particular, the 'Multipharm's Fluid Magnesia' product is labelled for use in young babies. It does not have maximum daily dosages on the label. It has not been put through the rigorous assessment required for medicines to be safely used in such circumstances. The Director-General believes that without these comprehensive safeguards, this product poses a safety risk to babies and young children.
The Multipharm's Vai Lafa and Multipharm's Vai Tane products pose a safety risk because they are not adequately labelled with instructions for use. These products have not been assessed and approved for the treatment of ringworm or fungal infections. Medicines sold in New Zealand must be correctly labelled and meet required standards for quality, safety and effectiveness.
The Vaikahi Mixture is intended for the treatment of constipation. The label has no instructions or warnings in English and the medicine could pose a safety risk if taken incorrectly. Medicines sold in New Zealand must be labelled in English. This product has not been assessed and shown to meet required standards for quality, safety and effectiveness.
"Consumers should immediately stop using the products and seek medical advice from their doctor if they, their babies or children have been unwell when using any of these products," said Mr McKernan.
This warning also applies to any other similar unapproved medicines that may be on sale in New Zealand. Medsafe is undertaking further investigations into the supply of medicines originating from the Pacific Islands and sold by retailers in New Zealand. Under the medicines legislation, retailers, distributors and importers are responsible for the products they sell and they must ensure that they have approval for any medicines they wish to sell prior to selling them. This ensures that all medicines for sale have been assessed for their quality, safety and efficacy and that they can be marketed lawfully in New Zealand.
ENDS
For further information please contact: Michael Flyger, Media Advisor, Ministry of Health ph 04 496 2265 or 027 434 6878
Photos
Vaikahi Mixture |
Multipharm's Fluid Magnesia |
 |
 |
Questions and Answers
What is wrong with these products?
All four products are being sold illegally as they have not been approved for sale through the New Zealand medicines approval process.
Multipharm's Fluid Magnesia is being sold for the treatment of 'wind and tummy pain' in babies and young children. The manufacturing standards for this medicine have not been assessed and so no assurance can be given about its quality. It is labelled with a three year expiry date which may be inappropriate because it may deteriorate during that time or become contaminated with bacteria during use.
Multipharm Vai Lafa and Multipharm Vai Tane contain salicylic acid in a flammable alcohol solution and they are intended for the treatment of ringworm and fungal infection. There are no instructions for use. These medicines have not been assessed and approved for the treatment of ringworm or fungal infections and may not be effective. The conditions under which these products have been manufactured have not been assessed so their quality cannot be guaranteed.
Vaikahi mixture has not been approved for sale in New Zealand and may not meet the required quality, safety and efficacy standards.
If a consumer is taking or using one of these products what should they do?
Consumers are being warned to immediately stop taking or using these products and seek medical advice from their doctor if they, their babies or children become unwell when using the products.
Adverse reactions to these products should be reported to the Centre for Adverse Reactions Monitoring: https://pophealth.my.site.com/carmreportnz/s/
Consumers can also report any concerns to Medsafe: www.medsafe.govt.nz/safety/report-a-problem.asp
Have these products been removed from sale?
It is possible that these products may still be on sale. Medsafe welcomes information about retailers who continue to sell them.
It is possible that other similar unapproved products may also be on sale. Medsafe welcomes any information from consumers about the sale of such products.
Medsafe is continuing to investigate the matter and may take further regulatory action if required.
Important advice to traders
Under the medicines legislation, manufacturers, distributors and importers are required to obtain approval before they sell or distribute products intended for a therapeutic purpose. Retailers cannot lawfully sell products that have not first been approved for sale under the provisions of the Medicines Act 1981.
Under section 20 of the Medicines Act 1981, Ministerial consent is required for the sale or distribution of new medicines in New Zealand.
A breach of this requirement carries substantial penalties.
An individual who sells, distributes or advertises the availability of any medicine without Ministerial consent is liable on conviction to imprisonment for a term not exceeding six months or a fine not exceeding $20,000.
A body corporate which sells, distributes or advertises the availability of any medicine without Ministerial consent is liable on conviction to a fine not exceeding $100,000.
The Ministry of Health takes breaches of the medicines laws very seriously, especially where patient and consumer safety is put at risk, and regulatory action will be taken as necessary to ensure that the law is complied with.





