Revised: 31 May 2017
Publications
Media Releases for 2008
11 December 2008
Health warning issued under Section 98 of the Medicines Act 1981 - Samoan Pharmacy Products
The Director-General of Health, Stephen McKernan, is warning the public about the potential health dangers associated with three unapproved medicines manufactured in a pharmacy in Samoa and sold from general retailers in New Zealand.
This statement about the three medicines is being issued by the Director-General under Section 98 of the Medicines Act 1981, following an investigation by the Ministry of Health's medicines safety arm, Medsafe.
The three medicines are labeled as originating from ‘Maria’s Healthcare Pharmacy Ltd’ in Apia. They have been imported by a wholesaler and distributed in New Zealand to various retail outlets.
The products are:
- 'Baby Magnesia', a product that is labelled as being suitable for babies as young as 3 months for the relief of 'wind and tummy pains'. The label states that the product contains the ingredients: citric acid, sodium bicarbonate and magnesium carbonate.
- 'Vai Lafa', a product intended for the treatment of ringworm. The label states that the product contains 15% Salicylic Acid.
- 'Vai Tane', a product intended for the treatment of fungal infections. The label states that the product contains 10% Salicylic Acid.
The safety, quality and efficacy of these medicines are unknown as they have not been assessed and approved for supply in New Zealand through the medicines approval process.
In particular, the ‘Baby Magnesia’ product is labelled for use in young babies and has not had the rigorous assessment required for medicines used in such circumstances. The Director-General believes that without these comprehensive safeguards, this product poses a safety risk to babies and young children.
The Vai Lafa and Vai Tane products pose a safety risk because they are not adequately labelled with instructions for use and these products have not been assessed and approved for the treatment of ringworm or fungal infections. Medicines sold in New Zealand must be correctly labelled and comply with safety requirements.
"Consumers should immediately stop using the products and seek medical advice from their doctor if they or their babies and children have been unwell when using any of these products," said Mr McKernan.
This warning also applies to other similar medicines that may be on sale in New Zealand. Consumers are advised not to buy any medicines that have an overseas pharmacy label, have a pharmacy label but are not being sold by a pharmacy or have a label that does not have instructions for use in English as these medicines will not have been approved for sale in New Zealand. Medsafe would welcome information from consumers about the sale of any such medicines being supplied in this manner.
ENDS
For further information please contact: Michael Flyger, Media Advisor, Ministry of Health ph 04 496 2265 or 027 434 6878
Photos
Baby Magnesia |
Vai Lafa |
Vai Tane |
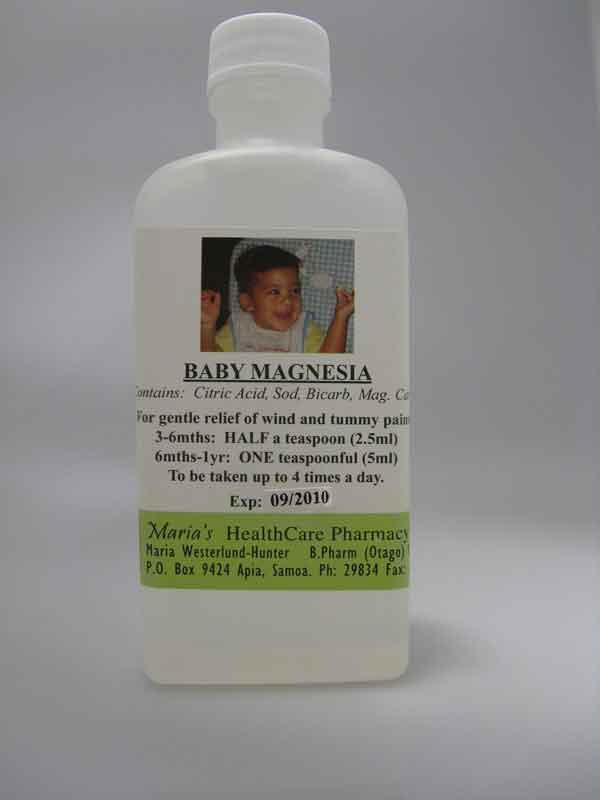 |
 |
 |
Questions and Answers
What is wrong with these products?
All three products are being sold illegally as they have not been approved for sale through the New Zealand medicines approval process.
Baby Magnesia is being sold for the treatment of 'wind and tummy pain' in babies and young children. The manufacturing standards for this medicine have not been assessed and so no assurance can be given about its quality. It is labelled with a two year expiry date which is inappropriate because it may deteriorate during that time or become contaminated with bacteria during use.
Vai Lafa and Vai Tane contain salicylic acid in a flammable alcohol solution and are intended for the treatment of ringworm and fungal infection. There are no instructions for use. These medicines have not been assessed and approved for the treatment of ringworm or fungal infections and may not be effective. The conditions under which these products have been manufactured have not been assessed so their quality cannot be guaranteed.
If a consumer is taking or using one of these products what should they do?
Consumers are being warned to immediately stop taking or using these products and seek medical advice from their doctor if they, or their babies or children have felt unwell when using the products.
Information so far indicates that approximately 520 bottles of Baby Magnesia and 130 bottles each of Vai Lafa and Vai Tane have been sold to consumers.
Adverse reactions to these products should be reported to the Centre for Adverse Reactions Monitoring: https://nzphvc.otago.ac.nz/report/
Consumers can also report any concerns to Medsafe: www.medsafe.govt.nz/safety/report-a-problem.asp
Have these products been removed from sale?
Stock distributed by one wholesaler and sold through retail outlets has been recalled from those outlets.
It is possible that other similar unapproved products may be on sale. Medsafe welcomes any information from consumers about the sale of such products.
Medsafe is continuing to investigate the matter and may take further regulatory action if required.
Important advice to traders
Under the medicines legislation, manufacturers, distributors and importers are required to obtain approval before they sell or distribute products intended for a therapeutic purpose. Retailers cannot lawfully sell products that have not first been approved for sale under the provisions of the Medicines Act 1981.
Under section 20 of the Medicines Act 1981, Ministerial consent is required for the sale or distribution of new medicines in New Zealand.
A breach of this requirement carries substantial penalties.
An individual who sells, distributes or advertises the availability of any medicine without Ministerial consent is liable on conviction to imprisonment for a term not exceeding 6 months or a fine not exceeding $20,000.
A body corporate which sells, distributes or advertises the availability of any medicine without Ministerial consent is liable on conviction to a fine not exceeding $100,000.
The Ministry of Health takes breaches of the medicines laws very seriously, especially where patient and consumer safety is put at risk, and regulatory action will be taken as necessary to ensure compliance.
5 December 2008
Health warning issued under Section 98 of the Medicines Act 1981 - Chinese Products
Director-General of Health Stephen McKernan is warning the public about the potential health dangers associated with six products sold from an Auckland supermarket selling Chinese goods.
This statement about the six products is being issued by the Director-General under Section 98 of the Medicines Act 1981, following investigations by the Ministry of Health's medicines safety arm, Medsafe.
The products are:
- Santi Scalper Penis Erection Capsules (Santi Nui Ban). This product contains the undeclared ingredients sildenafil (a prescription medicine used to treat erectile dysfunction).
- Masaone capsules. This product is labeled as containing the ingredient piroxicam, a potent anti-inflammatory medicine.
- Ankahuangmin Jiaonang (Paracetamol, Caffeine, Artificial Cow Bezoar and Chlorpheniramine Maleate Capsules). This product is presented as blister packs containing red/clear capsules with blue Chinese print on the foil backing and is labeled as containing chlorpheniramine maleate, an antihistamine used to treat allergies, and paracetamol used to treat pain and fever.
- Lufen Huang Min Pian (Compound Diclofenac Tablet). This product is presented in a silver tin containing silver foil blister packs each containing blue tablets. There is Chinese writing in black on the silver backing foil and this is labeled as containing diclofenac, an anti-inflammatory medicine, and chlorpheniramine maleate, an antihistamine used to treat allergies.
- Madame Pearl's Cough Syrup. This product is labeled as containing noscapine hydrochloride, a cough suppressant; phenylephrine hydrochloride, a decongestant; guaiphenesin hydrochloride, an expectorant and chlorpheniramine maleate, an antihistamine.
- Madame Pearl's Cough Syrup (for Children) is labeled as containing noscapine hydrochloride, a cough suppressant; guaiphenesin hydrochloride, an expectorant and chlorpheniramine maleate, an antihistamine.
The safety, quality and efficacy of these products are unknown as they have not been assessed for supply in New Zealand through the medicines approval process.
The Santi Scalper Penis Erection and Masaone products contain prescription medicines and should only be used on the advice of an authorised prescriber after the benefits and risks of their use have been assessed. The other products contain pharmacist or pharmacy-only ingredients and should only be sold from a pharmacy where a qualified healthcare professional is available for advice.
Medicines approved for sale without a prescription are required to be labelled in English with instructions on their intended purpose and dosage. Consumers are encouraged to report instances where labelling appears not to meet these requirements. Medicines for more serious conditions are only available from a pharmacy.
"Consumers should immediately stop taking these products and seek medical advice from their doctor if they are taking other medicines or if they have felt unwell when taking any of these products," said Mr McKernan.
Questions and Answers
What is wrong with these products?
The Santi Scalper Penis Erection Capsulesp contain the undeclared therapeutic substances, sildenafil and homosildenafil. Viagra is the only brand of sildenafil approved for sale in New Zealand and is used for managing erectile dysfunction. Sildenafil is known to interfere with some heart medications and could be fatal to some individuals. Sildenafil should only be used on the advice of an authorized New Zealand prescriber after the benefits and risks of use have been assessed.
More information about medicines that interact with sildenafil and other precautions relating to its use can be found by accessing the Consumer Medication Information on the Medsafe website by typing Viagra into the search engine at: www.medsafe.govt.nz//DbSearch/ .
The Masaone capsules contain piroxicam, a prescription medicine used as an anti-inflammatory for treating pain, swelling and inflammation due to musculo-skeletal conditions such as arthritis. Piroxicam can cause serious reactions, such as stomach upset, ulceration, and bleeding. Piroxicam can also interact with other medicines. As a result, piroxicam should only be used on the advice of an authorized New Zealand prescriber after the benefits and risks of use have been assessed.
More information on the effects of piroxicam can be found by accessing the Consumer Medication Information on the Medsafe website by typing piroxicam into the search engine at: www.medsafe.govt.nz//DbSearch/ .
The Lufen Huang Min Pian tablets contain diclofenac sodium and chlorpheniramine maleate in a presentation which would make the product a pharmacist only medicine. Diclofenac, like piroxicam, can also cause serious reactions and may interact with other medicines. Diclofenac should only be used on the advice of a heathcare professional.
The Ankahuangmin Jiaonang capsules and the Madame Pearl's products contain common cough and cold ingredients. These products should only be sold from a pharmacy where healthcare professional advice is available as they may not be suitable for some people who have certain health conditions or who are taking other medicines. In addition, the use of cough suppressants is not recommended in children under two years of age.
If a consumer is taking one of these products what should they do?
Consumers are being warned to immediately stop taking these products and seek medical advice from their doctor if they are taking other medicines or have felt unwell when taking the products. There is no reliable information about how many people have taken these products.
Adverse reactions to these products should be reported to the Centre for Adverse Reactions Monitoring: https://nzphvc.otago.ac.nz/report/. Consumers can also report any concerns to Medsafe: www.medsafe.govt.nz
Have these products been removed from sale?
Stock held by one retailer has been seized by Medsafe investigations staff. Some of the products have been sold by a wholesaler to several retail outlets. This stock has been recalled. Medsafe is continuing to investigate the matter and may take further regulatory action if required.
Have the products on sale in New Zealand been tested?
Santi Scalper Penis Erection Capsules have been tested and found to contain sildenafil and homosildenafil (an analogue of sildenafil that is believed to have similar therapeutic actions). The other products have not been tested because the ingredients are stated in English on the labels.
Where can I find more information about Viagra and its active ingredient and side effects?
Consumers seeking general information about Viagra and its active ingredient sildenafil can access the Consumer Medication information on the Medsafe website by typing the trade name of the product into the search engine at: www.medsafe.govt.nz//DbSearch/ .
What about other similar products for sexual enhancement?
Many products appear to be available from retailers and over the internet claiming to be for sexual enhancement, to improve sexual performance or for the treatment of erectile dysfunction. Because of the illicit international trade in these products it is not possible for anyone to be certain about the contents of any of the unapproved products on sale in New Zealand. Previous investigations by Medsafe have identified a number of supposedly natural products adulterated with medicines such as sildenafil.
The concern and risk of serious consequences (including death) is real enough for Medsafe to warn against taking any of these products.
Important advice to traders
Under the medicines legislation, sponsors, distributors and importers are required to obtain approval before they sell or distribute products intended for a therapeutic purpose. Retailers cannot sell products that have not first been approved for sale under the provisions of the Medicines Act 1981.
Section 20 of the Medicines Act 1981 requires medicines to be approved before distribution in New Zealand. A breach of this requirement carries substantial penalties.
On conviction, the maximum penalty for an individual who sells a medicine without first having it approved through the regulatory process administered by Medsafe is $20,000 or up to 6 months in prison.
On conviction, the maximum penalty for a body corporate which sells a medicine without first having it approved through the regulatory process administered by Medsafe is $100,000.
The Ministry of Health takes breaches of the medicines laws very seriously, especially where patient and consumer safety is put at risk, and regulatory action will be taken as necessary to ensure compliance.
Images of the products can be found on Medsafe's website at www.medsafe.govt.nz/hot/alerts/GL/Photos.asp
For further information, please contact Michael Flyger (MoH Media Advisor) DDI: 04 496 2265 / Mobile: 0274 346 878
13 November 2008
International Internet Day of Action against Counterfeit Medicines
New Zealand's drug regulatory arm, Medsafe, is participating in the International Internet Day of Action, aimed at raising awareness around the risks of self-diagnosis and subsequent buying of medicines over the Internet.
In the past six months Medsafe has seized 57 consignments of counterfeit versions of the erectile dysfunction medicines Viagra or Cialis at New Zealand borders. The products had been imported for personal use, and in nearly all cases the consumers who had purchased these medicines over the internet would have been unaware they had purchased a counterfeited medicine.
Viagra and Cialis are prescription medicines that can be obtained through New Zealand pharmacies and are readily available on prescription from a New Zealand-registered doctor.
"Medsafe recommends consumers avoid purchasing medicines over the internet. The practice of self prescribing and purchasing medicines in this way carries significant health risks," said Derek Fitzgerald, Medsafe's Manager Compliance.
"International investigations have revealed that these products are often of poor quality and made in unhygienic circumstances. In addition, testing of internet purchased counterfeit products has found they may contain anything from little or no active ingredient through to excessive quantities. In some circumstances, counterfeit products have also been found to contain other undeclared ingredients, such as medicines, that may be dangerous."
The sale of counterfeit medicines affects all countries and use of counterfeit medicines has led to a large number of deaths across the globe.
Background material
In June 2008 New Zealand became the 17th permanent member country within the Permanent Forum on International Pharmaceutical Crime (PFIPC).
This forum is endorsed by INTERPOL and operates the International Medical Products Anti-Counterfeiting Taskforce (IMPACT), an initiative jointly operated by the World Health Organisation.
Medsafe, the New Zealand Medicines and Medical Devices Safety Authority, represents the Ministry of Health on this important international forum.
The aim of the forum is to co-ordinate networks across and between countries in order to halt the production, trade and sale of counterfeit medicines and medical devices around the world.
12 November 2008 sees the first International Internet Day of Action undertaken by participating PFIPC countries.
The aim of this operation is to demonstrate there is an international will to address the problems associated with counterfeit medicines and to heighten the awareness of the risks associated with using medicines purchased over the internet.
New Zealand joins Australia, Belgium, Brazil, Canada, England, Germany, Israel, Italy, the Netherlands, Northern Ireland, the Republic of Ireland, Singapore, South Africa, Spain, Switzerland and the USA as part of an international team of regulators committed to heightening awareness and endeavouring to prevent this growing global phenomenon.
ENDS
For further information, please contact Michael Flyger, Media Advisor: 04 496 2265, 0274 346 878
23 October 2008
Medsafe approves two additional levothyroxine products
The Ministry of Health's drug regulatory arm Medsafe has given provisional approval to two products containing levothyroxine. This will allow patients in New Zealand to have access to an approved alternative to Eltroxin.
The products are Synthroid, manufactured by Abbott Laboratories, which is also sold in Canada, and Levothyroxine, manufactured by Goldshield, which is also sold in the United Kingdom.
The provisional consent limits use of the products to initiation of treatment in new patients, and use in patients who are intolerant or unable to take another brand of levothyroxine. The Medsafe data sheets for the products will reflect these restrictions and each company will be required to notify prescribers and pharmacists of the increased need to closely monitor patients who switch brands of levothyroxine.
Medsafe group manager Dr Stewart Jessamine says "Following our evaluation of the data supplied in the applications, Medsafe is satisfied that both Levothyroxine and Synthroid are safe and effective alternatives to Eltroxin, however as neither application provided adequate evidence of bioequivalence use of these products will require careful monitoring if patients wish to avoid adverse effects."
Dr Jessamine said: "while changing to a new levothyroxine product will resolve many of the problems reported by patients taking Eltroxin, patients changing to these new brands will still need to have their blood tests monitored and are likely to require the dose of the new brand adjusted to suit their individual metabolism".
PHARMAC Medical Director Dr Peter Moodie said the provisional consent given to the Goldshield brand means that it will be fully funded from 1 November. This was in line with the proposal PHARMAC consulted on from late September, he says.
The existing funded brand of levothyroxine, Eltroxin, will also continue to be fully funded.
PHARMAC has been able to put in place arrangements to ensure that when funding for Goldshield begins on 1 November, there will be sufficient stock to meet the expected demand.
"We are very grateful to the efforts of Goldshield's supplier, Boucher & Muir, for getting stocks to New Zealand rapidly," said Dr Moodie. "We understand that stock is in the country this week, and will be distributed to wholesalers next week. This will ensure that should people require a prescription for Goldshield's brand of levothyroxine to be filled from 1 November, they will be able to do so."
Dr Moodie says PHARMAC has no plans to fund Synthroid at this time, however it remains open to the possibility of funding it in the future.
Background
Full background to this issue, including a timeline, questions and answers
and previous media releases is available by clicking the following link:
www.medsafe.govt.nz/hot/MediaContents.asp
ENDS
25 September 2008
Voluntary quarantine of Ranbaxy drugs in place
New Zealand's reaction to the United States Food and Drug Administration import alert around three pharmaceuticals is in line with other regulators internationally. The alert, issued by the FDA last week, relates to products manufactured by Indian drug firm Ranbaxy Laboratories.
Medsafe has contacted other pharmaceutical regulators worldwide and while still waiting for further replies, it is clear that the United Kingdom, Germany, the Netherlands, Australia and Canada have not moved to restrict the availability of these products. The World Health Organisation has issued an advisory which details their actions, which are similar to moves underway in New Zealand. http://healthtech.who.int/pq/
Further information, including initial test results on the batches of the drugs already in New Zealand, is expected in about three weeks. The two suppliers of the three Ranbaxy funded products available in New Zealand, Douglas Pharmaceuticals Limited and Apotex New Zealand Limited have agreed to a voluntarily hold the products, which are due in the country in October. This means the products will be held in quarantine at the companies' own warehouses, and not released for distribution.
The focus on Ranbaxy's manufacturing processes arose when the United States imposed an import alert on medicines imported into the United States after FDA audits of two Ranbaxy sites in March this year. The auditors raised concerns over good manufacturing practices at the sites.
The voluntary quarantine of imported product in New Zealand will allow more time for testing of the products as well as allowing Medsafe to continue discussions with other international regulators.
Ministry Chief Advisor Public Health Dr Ashley Bloomfield says Medsafe has contacted regulators from the Netherlands, Germany, the United Kingdom, Canada and Australia. None are planning to restrict the availability of any of the listed products, through either an import ban or recall, advice which is consistent with and validates Medsafe's current approach. He says the voluntary action by local importers means a decision around a possible import ban can be deferred until the end of October, when more information will be available.
Dr Bloomfield says the advice to anyone concerned about the products remains the same. He says Medsafe's advice echoes that issued by the FDA and the WHO - there is no evidence that individual drugs have been affected and patients may jeopardise their health by stopping taking them.
The three products manufactured at these two Indian sites that are both licensed and funded in New Zealand are amoxicillin syrup (a widely prescribed penicillin-based antibiotic), aciclovir (an antiviral drug used to treat the herpes virus) and cefaclor (an antibiotic used to treat a wide range of infections).
Anyone with concerns about any of these products can call Healthline on 0800 611 116. A link to Medsafe's previous release, plus a series of questions and answers about the FDA action and the Ranbaxy products supplied in New Zealand, can be found here:
www.medsafe.govt.nz/hot/MediaContents.asp
Ranbaxy Laboratories Limited is a multinational drug manufacturer operating in 11 countries including the United States (where there are three plants). It has worldwide sales of $US1.6 billion. More information about Ranbaxy can be found on the company's website at www.ranbaxy.com
ENDS
18 September 2008
Medsafe and PHARMAC acting on concerns over Ranbaxy medicines
The Ministry of Health's drug regulatory arm Medsafe is seeking renewed assurance of 'Good Manufacturing Practice' after an India based pharmaceutical company failed a US Food and Drug Administration audit at two sites early in the year.
Medsafe is seeking information from other trusted regulators (in Australia, Europe, Britain and Canada) to see if other audits can provide up-to-date assurance about 'Good Manufacturing Practice' at the two sites operated by Ranbaxy Laboratories Limited.
Ministry Chief Advisor Public Health Dr Ashley Bloomfield says Ranbaxy is a major contract manufacturer for both generic and innovative medicine companies. Its medicines are supplied to many parts of the world and Ranbaxy is frequently audited. It's quite possible that other audits will have given the two sites a clean bill of health.
The US FDA issued its warnings and import alerts for the two affected manufacturing sites early Wednesday (New Zealand time) based on its audits carried out much earlier in the year. The FDA's move is expected to apply pressure on Ranbaxy to respond more quickly to the FDA's concerns.
Dr Bloomfield says there is provision for an import ban under the Medicines Act. Medsafe has initiated the process that would enable a ban to be placed on further imports to New Zealand if the assurance being sought from other trusted regulators is not provided. If required, the import ban would be put in place before the next consignment of Ranbaxy stock is due to arrive in the country.
As an added assurance, Medsafe will initiate a targeted programme of product testing to confirm the quality and safety of the Ranbaxy medicines made at the affected sites. Similar tests carried out in the US have revealed no problems and US authorities are advising that Ranbaxy medicines already in the United States should continue to be used with only imports to be stopped at the border.
Dr Bloomfield says in both countries the advice to individuals taking these medicines is the same - there is no evidence that individual drugs have been affected and patients are advised they should continue taking them.
In the United States one drug produced at the two sites affected by the import ban has been exempted from the import alert as there is no readily available alternative in the US. This product is not distributed in New Zealand.
Medsafe and drug funding agency PHARMAC are working together to ensure minimum disruption to medicine availability - if tougher regulatory action is necessary. As a contingency measure, PHARMAC is now looking at alternative supply arrangements should this be required.
PHARMAC Medical Director Peter Moodie says we have at least 3 months supply of the medicines affected. He says it’s timely to start looking now for any alternatives even though we may never need them its better to be prepared now as alternatives may take some time to source.
Dr Bloomfield says we know that the FDA do not have safety concerns around any individual product, nor do they have any reports of adverse events linked to the manufacturing process of the drugs. He says other generic and innovator medicines, manufactured by the same company at other sites in India are unaffected.
However the FDA says they found a number of deficiencies during their audit of the company's 'Good Manufacturing Practice'; enough to warrant an import alert for products manufactured at the affected sites, but not sufficient to result in a recall of product in the United States.
Three branded medicine products manufactured at these Indian sites are both licensed and funded in New Zealand. They are amoxicillin syrup (a widely prescribed penicillin-based antibiotic), aciclovir (an antiviral drug used to treat the herpes virus) and cefaclor (an antibiotic used to treat a wide range of infections).
Dr Bloomfield says amoxicillin is very frequently used in New Zealand. He says it is important that the FDA measures are seen in context and that people are not unnecessarily concerned.
"There is no evidence that amoxicillin syrup, or the other drugs affected, are either unsafe or ineffective. All health professionals have been made aware of the situation and will be able to advise on options, if people have concerns."
Medsafe advises that anyone with concerns about these medicines should in the first instance call Healthline on 0800 611 116 or talk to their health professional.
Medsafe will be keeping in close contact with its Australian counterpart the Therapeutic Goods Administration and other regulators as well as keeping a close eye on any further regulatory action by the US FDA.
For more information contact:
Michael Flyger, Ministry of Health Communications Advisor 0274-346-878
Simon England, PHARMAC Communications Manager 021-863-342
For further information, please contact Michael Flyger (MoH Media Advisor) 04 496 2265 / 0274 346 8781
11 September 2008
Eltroxin
The Ministry of Health's drug regulatory arm Medsafe and Government drug-funding agency PHARMAC are well advanced in their efforts to source an alternative brand to the funded thyroid medicine Eltroxin.
Medsafe Group Manager Dr Stewart Jessamine says on September 3, Medsafe received its first application to seek approval for another brand of levothyroxine and he has been assured that an application for a second alternative is due to be submitted in the next week.
PHARMAC Funding and Procurement Manager Steffan Crausaz says PHARMAC will be able to give an update on funding an alternative within the next two weeks.
Since May of this year, when the numbers of adverse reactions to Eltroxin began to significantly increase, Medsafe has been contacting manufacturers and distributors of medicines encouraging them to submit an application for alternative brands of levothyroxine. "While any new products will still need to meet the required standards of safety, quality and efficacy before they can be approved, Medsafe will fast track the assessment of any applications it receives for levothyroxine to allow an approved alternative to be made available as promptly as possible," Dr Jessamine said.
Steffan Crausaz says approval by Medsafe is only the first step in the process of making a funded alternative to Eltroxin available and PHARMAC expects to complete negotiations with at least one of the alternative medicine supply companies shortly.
PHARMAC has been holding discussions with suppliers in parallel with Medsafe's work, and once a medicine has Medsafe approval, PHARMAC is well placed to move quickly on funding, says Steffan Crausaz.
"We need to be assured that sufficient stock will be available, and to consult with suppliers and the public on the listing arrangements."
Dr Jessamine says in the meantime the advice received from specialist endocrinologists is that many of the adverse reactions are consistent with some patients absorbing lower amounts of levothyroxine from the new tablets. In their expert experience, blood tests to check the level of thyroid hormone stimulating hormone (TSH), and adjustment of the dose of Eltroxin, either to increase the dose (or in some cases to decrease it) will lead to resolution of the patients symptoms over a few weeks to months. Patients taking Eltroxin who are worried about the product, or who have already changed to other brands of unapproved levothyroxine, should contact either Healthline on 0800 611-116 or their doctor, as they may need ongoing thyroid blood monitoring and dose adjustment.
Dr Jessamine confirmed the results of independent testing of both the old and new formulation of Eltroxin by ESR indicate the new Eltroxin tablets are acceptably potent and do not contain unexpected or excessive impurity content, compared to the old formulation, and meet the requirements for dissolution (a marker for how quickly the products dissolve). The batches of products tested include samples obtained from a pharmacy and from Eltroxin manufacturer GlaxoSmithKline (GSK).
Dr Jessamine says some of the claims being circulated about the product are wrong, such as:
- it is not manufactured in India
- its manufacture does not involve any genetic engineering
- it does not contain MSG or any products not found routinely in other medicines.
The active ingredient (levothyroxine) in the new formulation is made in Austria by the same company, using the same method at the same site as the old formulation, and the finished product is manufactured in Germany. The other ingredients of the new formulation are routinely found in a range of other medicines and are not associated with increased rates of adverse effects.
The new GSK formulation of Eltroxin is currently marketed in close to 30 countries around the world. Medsafe has sought information from several of these countries where they have comparable adverse reactions reporting systems to New Zealand and it is clear they are not receiving increased numbers of reports for the new formulation product.
Medsafe also asked each of the 83 countries who make up the World Health Organisation Adverse Reactions Reporting System for any information they had on increased rates of reporting to GSK Eltroxin, or to adverse reactions following switches to brands of levothyroxine. The only positive feedback received came from the United Kingdom and Australia, countries where the GSK brand of Eltroxin is not available, who reported that they have small numbers of reports of adverse reactions that are similar in nature to those received in New Zealand but associated with patients shifting between different brands of levothyroxine.
While most patients (approx 99%) continue to have no major problems with the GSK Eltroxin, many of the side effects reported by the 800 individuals who have submitted reports to the New Zealand Pharmacovigilance Centre would be explained by changes in how these individuals absorb, metabolise or excrete the new formulation of Eltroxin compared to the old product.
Dr Jessamine says that while changing to a new levothyroxine product may resolve some of the problems where the person is allergic to, or intolerant of, the new formula of Eltroxin, patients will still need to have their blood tests monitored and it is quite likely that they will need to have the dose of any new brand of product adjusted to suit their individual metabolism. If patients wish to avoid adverse effects associated with increased or decreased thyroid activity that can occur following a switch in brands, so careful monitoring and dosage is essential.
For more information contact:
PHARMAC Communication Manager Simon England 021-863-342
Ministry of Health Media Advisor Michael Flyger 027-434-6878
Background
Hypothyroidism
Thyroid medicines contain the active ingredient levothyroxine – a thyroid hormone.
Levothyroxine is used to treat hypothyroidism, a disease in which the thyroid gland is underactive and does not produce enough thyroxine, a hormone, which is important for controlling your metabolism.
Symptoms of hypothyroidism include tiredness, muscle weakness, cramps, feeling the cold, a slow heart rate, dry and flaky skin, hair loss, a deep husky voice and weight gain.
Children and elderly people usually need a smaller dose because they are more sensitive to the effects of levothyroxine.
Many people need treatment with levothyroxine long term.
Levothyroxine is produced in tablet form. Tablets are swallowed whole with a glass of water. Tablets are taken on an empty stomach, 30 minutes before breakfast.
Rare reactions to levothyroxine include:
- Diarrhoea
- Vomiting
- Palpitations (irregular heartbeat)
- Chest pain
- Sweating/flushing
- Weight loss
- Muscle weakness/cramps, tremors
- Rapid breathing
- Fever
- Headache
- Inability to sleep
- Feeling restless/excited
Many of these side effects often disappear when the dose is adjusted (lowered).
Patient advice:
Medsafe advises patients taking thyroxine to follow the dose instructions carefully and to contact their pharmacist or GP to discuss any concerns or questions they may have about this medicine.
Medsafe encourages GPs and pharmacists to review how patients are taking this medication, to monitor the effects of the change to the new formulation and to report any side effects, including problems with maintaining adequate control of hypothyroidism, to the New Zealand Pharmacovigilance Centre at the University of Otago. It is important that patients who report any adverse effects have their blood tests checked at the time of reporting. Most reports received so far have not included information about blood test results.
Although the new formulation of Eltroxin has been approved as safe and effective by Medsafe; prescribers, pharmacists and patients need to bear in mind that each individual may respond differently to the new formulation and that patients taking it should be monitored by a health professional and dose adjustments made if necessary. Patients should also make sure they are taking their medication on an empty stomach and are taking whole tablets in accordance with the advice from their GP.
The Medsafe data sheet on Eltroxin is available here:
www.medsafe.govt.nz/Profs/Datasheet/e/Eltroxin(new)tab.pdf
Testing
Testing by ESR has confirmed that Eltroxin contains nothing other than ingredients specified on the label:
- Levothyroxine sodium hydrate
- Magnesium stearate
- Microcrystalline cellulose
- Pregelatinised maize starch
- Purified talc
- Silicon dioxide
GSK no longer manufactures the formulation of levothyroxine previously sold in NZ, and no changes in formulation are planned.
The GSK brand of levothyroxine has been the only brand of product available and supplied in New Zealand for a number of years.
Prescribers can source thyroxine medicines direct from overseas under an exemption of the Medicines Act, but the medicines sourced this way have not been assessed or funded and require informed consent from patients.
GlaxoSmithKline's contact details are as follows:
GlaxoSmithKline NZ Limited
AMP Centre
Cnr Albert & Customs Streets
Private Bag 106600
Downtown
Auckland
NEW ZEALAND
Telephone (09) 367 2900
Facsimile (09) 367 2506
ENDS
For further information, please contact Michael Flyger (MoH Media Advisor) 04 496 2265 / 0274 346 878
8 August 2008
Health warning - Erectile dysfunction / sexual enhancement 'herbal' products
Director-General of Health, Stephen McKernan, is warning people about the potential health dangers associated with three products promoted and sold in New Zealand for sexual enhancement or the treatment of erectile dysfunction which may contain an undeclared therapeutic substance.
This statement about the three products is being issued by the Director-General under Section 98 of the Medicines Act 1981, following investigations by the Ministry of Health's medicines safety arm, Medsafe.
The products are Rize 2 the Occasion (also known as Rize 2), Rose 4 Her and Viapro. The products appear to have been sold by retail from ‘adult’ shops and over the internet.
The United States FDA has issued a warning that products on the US market with these names had been tested and recalled after they were found to contain the substance thiomethisosildenafil which is an analogue of sildenafil. Thiomethisosildenafil is expected to have similar therapeutic actions and adverse effects as sildenafil the active ingredient of the prescription medicine Viagra. Sildenafil is known to interfere with some heart medications and its use could be fatal to some individuals.
"Consumers should immediately stop taking Rize 2 the Occasion, Rose 4 Her and Viapro and seek medical advice from their doctor if they are taking other medicines or if they have felt unwell when taking any of these products," said Mr McKernan.
Stephen McKernan also warned that Medsafe has previously identified a number of other products being sold in shops and over the internet to treat erectile dysfunction or for sexual enhancement that have also been adulterated with prescription medicines. Consumers should treat erectile dysfunction products offered for sale without a prescription with caution and seek medical advice before using them.
Under the medicines legislation, sponsors, distributors and importers are responsible for the products they sell and must be aware of all the active ingredients they contain and seek approval prior to selling them if required by the legislation.
ENDS
For further information please contact:
Michael Flyger, Media Advisor, Ministry of Health ph 04 496 2265 or 027
474 6878
Questions and Answers
What is wrong with these products?
The products may contain an undeclared therapeutic substance which is expected to be similar in effect and in its adverse reactions to sildenafil. Sildenafil is the active ingredient in Viagra.
Viagra is the only brand of sildenafil approved for sale in New Zealand and is used for managing erectile dysfunction. Sildenafil is known to interfere with some heart medications and could be fatal to some individuals. Products containing substances similar to sildenafil should only be used on the advice of an authorized New Zealand prescriber after the benefits and risks of use have been assessed. More information about medicines that interact with sildenafil and other precautions relating to its use can be found by accessing the Consumer Medication Information on the Medsafe website by typing Viagra into the search engine at: www.medsafe.govt.nz//DbSearch/infoSearch.asp .
The safety, quality and efficacy of the products in question is unknown as they have not been assessed for supply in New Zealand through the medicines approval process.
If a consumer is taking one of these products what should they do?
Consumers are being warned to immediately stop taking these products and seek medical advice from their doctor if they are taking other medicines or have felt unwell when taking the products.
There is no reliable information about how many people have taken these products.
Adverse reactions to these products should be reported to the Centre for Adverse Reactions Monitoring: https://nzphvc.otago.ac.nz/report/.
Consumers can also report any concerns to Medsafe: www.medsafe.govt.nz
Have these products been removed from sale?
Distributors and retailers are warned that they should cease selling these products as they cannot be sure about what they contain and their sale may be in breach of the medicines legislation. Medsafe is contacting 'adult' shops and websites requiring the removal of these products from sale and providing general information about responsibilities retailers have under the legislation with respect to selling these types of products.
Medsafe is continuing to investigate the matter and may take regulatory action if necessary.
Have the products on sale in New Zealand been tested?
It is not currently possible to test for thiomethisosildenafil in New Zealand. Thiomethisosildenafil is a newly detected adulterant being added to supplements promoted for the treatment of erectile dysfunction and for sexual enhancement purposes. Information from the manufacturers and suppliers about the content of erectile dysfunction products sold over-the-counter or by internet is likely to be inadequate so it is not possible to rely on the labels or other statements made about the ingredients in these products.
Where can I find more information about Viagra and its active ingredient and side effects?
Consumers seeking general information about Viagra and its active ingredient sildenafil can access the Consumer Medication information on the Medsafe website by typing the trade name of the product into the search engine at: www.medsafe.govt.nz//DbSearch/infoSearch.asp
What about other similar products?
Many products appear to be available from retailers and over the internet claiming to be for sexual enhancement, to improve sexual performance or for the treatment of erectile dysfunction. Because of the illicit international trade in these products it is not possible for anyone to be certain about the contents of any of the unapproved products on sale in New Zealand. Previous investigations by Medsafe have identified a number of products adulterated with medicines such as sildenafil.
The concern and risk of serious consequences (including death) is real enough for Medsafe to warn against taking any of these products.
Important advice to traders
Under the medicines legislation, sponsors, distributors and importers are required to obtain approval before they sell or distribute products intended for a therapeutic purpose.
Section 20 of the Medicines Act 1981 requires medicines to be approved before distribution in New Zealand. A breach of this requirement carries substantial penalties.
On conviction, the maximum penalty for an individual who sells a medicine without first having it approved through the regulatory process administered by Medsafe is $20,000 or up to 6 months in prison.
On conviction, the maximum penalty for a body corporate which sells a medicine without first having it approved through the regulatory process administered by Medsafe is $100,000.
The Ministry of Health takes breaches of the medicines laws very seriously, especially where patient and consumer safety is put at risk, and regulatory action will be taken as necessary to ensure compliance.
Links to FDA warnings
- http://www.fda.gov/oc/po/firmrecalls/devine07_08.html
- http://www.fda.gov/oc/po/firmrecalls/eglabs07_08.html
Link to Health Canada warning
23 July 2008
Update on New Zealand contaminated Heparin
Please attribute to Stewart Jessamine, Group Manager, Medsafe
Medsafe and the Ministry of Health are continuing to monitor the situation with respect to the global problem of heparin contaminated with over-sulphated chondroitin sulphate (OSCS) entering the market. Medicines regulators around the world have taken a number of different approaches to managing this issue. In New Zealand, Medsafe requires all manufacturers of heparin-based products to test for the presence of OSCS in products supplied to the market. To date all of the manufacturers of heparin and low molecular weight heparin products supplied to New Zealand have reported that OSCS has not been detected in the products used by patients.
In response to reports that some batches of Clexane that have tested negative for OSCS have been made using heparin that contained a low level of OSCS, Medsafe and its expert advisory committee reassessed the safety of these batches and concluded that they could continue to be distributed in New Zealand. In addition, Medsafe has further tightened its controls on manufacturers by requiring that all ingredients used in the manufacture of heparin and low-molecular weight heparin must be negative for OSCS.
Medsafe's investigation has identified only one medical device that is used in direct contact with patients which has utilised heparin containing OSCS as a coating. The suppliers of this device which is used in cardiac surgery have already issued advice to specialists using the device advising that alternative products should be used if possible. Uncontaminated supplies of the device are not expected to be available for another two months.
While there has been no increase in reporting of adverse reactions to heparin or low molecular weight heparins in New Zealand since the issue of OSCS contamination was first identified, Medsafe is advising medical professionals and DHBs, that even with the current testing systems it cannot assure that very low levels of OSCS are not present in some products. Medsafe continues to encourage healthcare practitioners to be on the look out for possible adverse reactions to heparin containing products and to report them to the New Zealand Pharmacovigilance Centre at the University of Otago.
Background
Key points:
- Detectable levels of over-sulphated chondroitin sulphate (OSCS) have not been found in medicines containing heparin or low molecular weight heparins supplied in New Zealand.
- Because of the limitations of the available test methods, the presence of low levels of OSCS in products cannot be completely excluded.
- There has been no increase in adverse reaction reports in New Zealand for medicines containing heparin or low molecular weight heparins.
- Two types of medical device have been found to contain heparin contaminated
with OSCS. These are:
- an oxygenator used in cardiac surgery, where alternative products should be used if possible
- blood collection containers, where use of the products has been allowed where OSCS levels are below 6%.
- Healthcare practitioners are asked to be alert for, and to promptly report, any adverse reactions occurring in patients exposed to medicines or medical devices containing heparin or low molecular weight heparins.
General
Early this year the United States Food and Drug Administration (FDA) advised of serious adverse reactions in the USA associated with the intravenous use of certain brands of heparin injections. Further investigation identified that a contaminant, over-sulphated chondroitin sulphate (OSCS), was responsible for these adverse reactions.
The reactions were severe in some cases and were typically immunoallergic in nature (anaphylactic type reactions). By mid-April 2008, approximately 81 deaths had been reported as associated with intravenous heparin containing high levels of OSCS.
As a result of the FDA reports, Medsafe immediately conducted a review of products available in New Zealand and issued advice to key stakeholders. Investigation of the problem has been ongoing.
Medicines
All medicines containing heparin or low molecular weight heparins1 supplied in New Zealand have been tested. In all cases, OSCS has been reported as 'not detected'. The limitations of the test method mean that OSCS levels below 1% will not be detected. Consequently, the presence of a low level of OSCS in products supplied in New Zealand cannot be completely excluded.
An analysis conducted by Medsafe, and endorsed by its expert advisory group, supports the conclusions of medicines regulators in Europe and the United States that it is safe to use products where OSCS has not been detected. International data demonstrate that there has been no increase in adverse reactions reported worldwide for heparin or low molecular weight heparin-containing medicines in which the presence of OSCS was 'not detected'. This is supported by New Zealand data which confirm there has been no increase in the reporting of adverse reactions to heparin or low molecular weight heparins in New Zealand in 2008.
Medical devices
Information so far indicates that only one medical device used in direct patient contact that utilises heparin contaminated with OSCS has been supplied in New Zealand. Users of this device, the Medtronic Trillium Oxygenator (used in cardiac surgery), have been informed of the need to use this device with caution and only where an alternative product is unavailable. Medtronic advises that it will be approximately two months before uncontaminated stock of the Trillium Oxygenator will be available.
Some blood sample containers have also been found to contain heparin contaminated with OSCS. Medsafe is satisfied that the presence of OSCS at levels less than 6% will not affect blood test results. Laboratories and other organisations using these products have been advised to be alert to the possibility of unusual test results when these tubes are used and to re-test if necessary.
Reporting of adverse reactions
Health practitioners should remain alert to the possibility of adverse events and should promptly report any adverse reactions appearing to be associated with medicines or medical devices containing heparin or low molecular weight heparin.
- Reports of adverse reactions to medicines should be sent to the New Zealand Pharmacovigilance Centre / Centre for Adverse Reactions Monitoring (CARM): www.medsafe.govt.nz/profs/adverse/reactions.asp
- On the Medsafe website there are forms for reporting adverse events and quality issues: (www.medsafe.govt.nz/regulatory/DevicesNew/9AdverseEvent.asp)
For further information, please contact Michael Flyger (Media Advisor)
04 496 2265 / 0274 346 878
- This includes batches of Clexane recalled in Australia where samples of the heparin used in the manufacture of some batches of enoxaparin (the active ingredient in Clexane) were found to contain OSCS. Testing of the enoxaparin in these batches for OSCS was negative.
27 June 2008
Eltroxin formulation change – Monitor patients and adjust dosing if necessary
Medsafe advisory
Eltroxin formulation change – Monitor patients and adjust dosing if necessary
Please attribute to Dr Stewart Jessamine, Interim Manager, Medsafe
Medsafe is writing to provide information and advice on the change in formulation of Eltroxin (levothyroxine, also known as, thyroxine) tablets which has led to a number of patients reporting problems such as sore eyes, palpitations, and headaches.
In response to these reports, Medsafe has reassessed the change in Eltroxin formulation and can confirm that the new formulation satisfies all quality, safety, and bioequivalence criteria. In addition, all excipients and excipient quantities present in the new formulation are commonly used in medicines.
Treatment of thyroid dysfunction is subject to significant inter-patient variability. Therefore, when patients are changed to new formulations or brands of levothyroxine, thyroid function monitoring is required to ensure the correct dose is being prescribed. Small changes in dosing of levothyroxine can affect serum thyroid hormone levels.
When changing a patient to the new Eltroxin formulation, Medsafe advises that:
- Thyroid function should be monitored to ensure each patient is being prescribed the correct dose. This is particularly important for patients who have noticed symptoms since changing to the new formulation. Recent adverse reaction reports have shown raised TSH levels in some patients, confirming the need for monitoring.
- When monitoring Thyroid function:
- Thyroid Stimulating Hormone (TSH) levels are the best indicator of thyroid function. If there are particular concerns about thyroid function, free T4 and free T3 levels should also be monitored.
- Thyroid function tests can be performed at any time of the day.
- Due to the long half life of levothyroxine (5 - 7 days), Thyroid function tests should be conducted no earlier than 4 – 6 weeks following a change in dose, or a change in formulation.
- Dose adjustments should not usually exceed 50 micrograms per day. Dose adjustments in the elderly, in patients with pre-existing heart disease, or in patients with diabetes should not exceed 50 micrograms on alternate days.
- Specialist advice should be sought if dose adjustments are required in children.
Additional factors to consider when prescribing Eltroxin tablets:
- Poor patient compliance should be considered as a possible cause of adverse effects. Compliance may be affected by the need to take Eltroxin tablets on an empty stomach, or the need for alternate day dosing in some patients.
- Adverse reactions should be reported to the Centre for Adverse Reactions Monitoring (CARM, Box 913, Dunedin. carmnz@otago.ac.nz). Please include, where possible, the patient’s TSH, T3, and T4 results, the current dosing regimen, and if and when the patient was changed to the new formulation.
- The Eltroxin data sheet includes dosing guidelines for transferring patients to the new Eltroxin formulation (www.medsafe.govt.nz/profs/Datasheet/e/Eltroxin(new)tab.htm ).
- The GlaxoSmithKline letter dated 18 June 2008 provides further information on changing patients to the new formulation (www.medsafe.govt.nz/hot/alerts/Eltroxin.pdf).
For further advice relating to Eltroxin tablets, please contact the manufacturer (Glaxo Smith-Kline) on 0800 808 500 (Monday to Friday 11 am – 6pm).
The new formulation of Eltroxin is the only brand of levothyroxine that has ministerial consent for distribution in New Zealand. Medsafe does not have any applications for levothyroxine tablets from any other pharmaceutical companies. Unapproved medicines (which includes other brands of levothyroxine) can only be supplied under provisions in the Medicines Act (Section 25 and 29) that require an authorised prescriber to request or obtain the medicine for a specific patient under their care. For further information on the use of unapproved medicines in New Zealand, please see: www.medsafe.govt.nz/regulatory/unapproved.asp.
ENDS
Link to Medsafe Dear Dr Letter
23 May 2008
Heparin
An expert advisory committee has endorsed the Ministry's approach to managing the risk posed from contaminated heparin used in a coating in medical devices.
The expert group was convened yesterday to provide expert advice to the Ministry's medicines and medical devices regulatory arm Medsafe. The committee endorsed the actions taken to date by Medsafe.
Medsafe's enquiries have identified six different types of medical devices available within New Zealand that result in contact with patients. Manufacturer testing of these devices has identified only one product that is coated with contaminated heparin.
This device is called the Trillium Affinity NT Hollow Fibre Oxygenator and is used in heart-lung bypass surgery.
Medtronic, the manufacturer of the affected product, last week advised clinicians to quarantine the product and where stocks permit to use uncontaminated product. The company estimates it will be 2-3 months before replacement stock of uncontaminated product is available.
In the interim Medsafe and the Advisory committee's consensus advice is that where no alternatives products are available, affected products should still be used. The benefits of using the Trillium Affinity Oxygenator continues to outweigh the very small risk of severe allergic-type reactions that have been reported in association with injectable forms of contaminated heparin.
Medsafe and the Advisory Committee reviewed a risk assessment performed by Medtronic.
The assessment reports that the device contains very small amounts of heparin within its coating. The risk analysis also reports that laboratory testing of the device when it was in-use found that no contaminant was released.
Furthermore, Medsafe agrees with this risk analysis that even in a worst case scenario, where all of the contaminant potentially present within the device coating was released, the levels of contaminant that would be found in the patients bloodstream would be too small to activate the specific chemical messaging system that is associated with the severe allergic-type reactions reported with injectable contaminated heparin.
"Although we believe the risk of severe allergic-type reactions occurring with medical devices containing contaminated heparin is likely to be very small. Clinicians and staff involved in using any medical device containing heparin have already been informed about the issue of potential contamination. This means that the equipment will be used with appropriate precautions. These staff have also been advised to report possible side effects that may be associated with use of these medical devices", Dr Jessamine said.
Please note - Dr Jessamine is only available for interviews this afternoon (Friday 23 May)
Background
Contamination with oversulfated chondroitin sulfate has affected the global supply of heparin. It has been established that no contaminated heparin injection product has been distributed in New Zealand, and measures are in place to ensure that no contaminated product enters the supply chain here.
Injectable heparin products contaminated with oversulfated chondroitin sulfate have been associated with serious anaphalactoid and allergic-type adverse reactions and deaths internationally. Research has indicated that these reactions occur due to the activation of a specific chemical messenger system associated with allergic-type reactions and that a certain minimum plasma (blood) level of contamination is required to activate this chemical pathway.
Medsafe has not received any reports of adverse reactions definitively caused by the use of heparin-containing medical devices. However, the level of reassurance this provides is limited by contaminated devices only recently entering the supply chain and the small number that have been used.
An example of a medical device that is in patient contact and contains heparin is a cardiac oxygenator. It has a coating of heparin bonded to the surface, to prevent the coagulation (clotting) of blood being passed through the machine during cardiac surgery. There is no test that can be performed on a medical device to determine whether or not it contains contaminated heparin. This determination can only be made by tracing and testing the batch of heparin used in the manufacture of the medical device.
Heparin is also used in some brands of test tubes and containers used to collect blood for testing in biochemistry laboratories. Manufacturer testing of these have indicated that several brands contain contaminated heparin. Preliminary research by these manufacturers however report that the presence of oversulfated chondroitin sulfate at levels of contamination up to 1% is not associated with clinically significant changes in the accuracy of the results obtained for close to all routine blood tests.
This is an international problem and Medsafe is working with overseas regulators to identify the scope of the problem and take appropriate action. Further information will be provided as it becomes available.
In response to this emerging safety issue Medsafe has taken a number
of actions including:
convening an expert group to provide practical, clinical, ethical and scientific
advice to the Director-General of Health in relation to the identification,
assessment and management of risk associated with contaminated heparin that
has been, or may have been, used in the manufacture of medical devices supplied
in New Zealand;
working closely with international agencies to ensure New Zealand is taking
similar steps to those taken overseas to protect patients;
alerting manufacturers, suppliers and importers of medical devices to the
issue of use of contaminated heparin and requiring them to supply Medsafe
with any information they hold about the safety of the heparin they have
used to enable Medsafe to introduce risk management strategies around the
possible further import of contaminated devices; and
communicating with health professionals to ensure they are aware of the
best advice on the issue as it comes to hand.
Further information
Further information on this issue can be found at:
www.medsafe.govt.nz
www.fda.gov/cdrh/safety/heparin-device-list.html (FDA list of affected
devices)
www.fda.gov/cdrh/safety/heparin-notice.html (FDA notice to medical device
manufacturers and distributors)
ENDS
What has the heparin become contaminated with?
The heparin has become contaminated with oversulfated chondroitin sulfate.
What is oversulfated chondroitin sulfate?
Oversulfated chondroitin sulfate mimics heparin's qualities and is a modified form of chondroitin sulfate. Chondroitin sulfate is a naturally occurring substance made from animal cartilage and is often used in supplements to treat arthritic joints.
How does this affect its safety and/or effectiveness of treatments?
Injectable heparin contaminated with this ingredient has caused severe
adverse reactions (anaphylaxis and hypotension) and deaths internationally.
Injectable heparin contaminated with oversulphated chondroitin sulfate is
not used in New Zealand.
The risk to patients is regarded as very low. There have been no reports
in New Zealand or internationally of harm conclusively linked to use of
a medical device containing the contaminated heparin.
In most cases the heparin found in medical devices is bound to the surface of the devices as a coating designed to stop blood clotting on the devices itself.
As only small amounts of heparin are used in these devices and the heparin
coating is not designed to dissolve away, any contaminant that may be present
would not be expected to be released into the patient's bloodstream.
It is unclear what effect on treatment the contaminated heparin will have.
Are other heparin products affected - for example heparin supplied in syringes or saline?
No, these are medicines and there no contaminated medicine has been supplied in New Zealand.
What is heparin used for in medical devices?
It is applied as a coating to prevent blood clotting. The coating designed to stick to the product and not to dissolve.
What alternatives, if any, are available?
There are other products from other manufacturers which may potentially be used; however it is not known whether the fittings on these devices are compatible with other devices needed to carry out surgery. Further research is being carried out to establish whether these products can be used.
How long until we get alternative supplies of contaminant-free heparin coated devices?
Indications from Medtronic, the manufacturers of the devices containing contaminated heparin are that it will be at least 2-3 months before stocks of medical devices containing uncontaminated heparin are available.
Who is Medsafe working with to ensure as much as possible is known about the problem?
Medsafe is working with regulators around the world including the US Food and Drug Administration. A Technical Advisory Group is being convened to provide expert clinical advice on the issue.
9 May 2008
Medsafe prosecutes South Auckland Traditional Chinese Medical Ltd
The Ministry of Health's medicines and medical devices regulator Medsafe is pleased with the result of a prosecution brought against South Auckland Traditional Chinese Medical Ltd and its directors.
The defendants, David Wang, Helen Zhang and their company, were fined a total of $45,000 plus costs in the Manukau District Court on 4 April having pleaded guilty in September last year to various charges under the Medicines Act 1981.
The charges were laid after a Ministry investigator noticed a sign at the South Auckland Chinese Medical Centre in Otahuhu, Auckland advertising "Natural Viagra", in February 2006. A purchase of the 'Natural Viagra' was made and the finding that it contained an undeclared prescription medicine resulted in an investigation.
Several unapproved medicines were found, including products containing undeclared prescription medicines tadalafil, sildenafil and sibutramine. Cialis is the only product containing tadalafil that is approved for distribution in New Zealand, Viagra is the only approved product containing sildenafil for use in erectile dysfunction and Reductil is the only approved product containing sibutramine. Several products indicated for a variety of conditions containing prescription medicines used for the treatment of asthma, glaucoma and eye infections were also seized.
"This result sends a strong message to those trying to import or sell products containing prescription medicines without a legal right to do so, therefore putting public safety at risk," said Derek Fitzgerald, Medsafe Team Leader, Compliance.
"This case demonstrates that consumers simply can't be assured about the safety or quality of the "natural" health product they are purchasing."
He says the defendants were claiming to sell a "natural" medicine which includes a perception that the product is safe, when in fact they were selling a prescription medicine which had the potential to cause serious harm.
"It's vital we send signals to the community of retailers selling natural medicines that they risk prosecution if they sell products containing pharmaceuticals and the onus is on them to make sure the products they sell comply with the law."
"Medsafe has increased its surveillance at the border to decrease the importation of unapproved medicines, and will take steps to prosecute companies and individuals selling products that contain pharmaceuticals where these products have been imported and / or distributed illegally", said Derek Fitzgerald.
Background and drug information
During its investigation Medsafe seized several products from the premises of South Auckland Traditional Chinese Medical Ltd, and found that they contained prescription medicines. Two contained the substance sildenafil - the active ingredient present in Viagra to treat erectile dysfunction, one contained tadalafil, the active ingredient present in Cialis to treat erectile dysfunction and one other contained sibutramine, the active ingredient in Reductil, a medicine used to treat obesity.
Sildenafil has known serious risks which affect the circulatory system. Its use is contraindicated in patients with certain cardiac conditions. Information available on the Medsafe website indicates that patients should not take Viagra in certain circumstances, including the following situations: (a) when being treated with a certain group of medicines (nitrates) for angina or other heart conditions; (b) when sexual intercourse would be inadvisable due to heart or blood vessel problems; (c) following a stroke or heart attack in the previous six months; and (d) when suffering from severe liver problems and (e) when blood pressure is unusually high or low and not effectively treated.
Tadalafil is also contraindicated in patients with certain cardiac conditions.
Sibutramine can cause increased blood pressure and heart rate and cannot safely be taken by a range of people, including those with glaucoma, mental illness and severe liver or kidney problems. It should not be used in combination with other medicines such as some antidepressants and migraine treatments.
The charges involved: advertising the availability of an unapproved medicine; selling an unapproved medicine; sale of prescription medicines otherwise than by a pharmacist in a pharmacy and the possession of prescription medicines without reasonable excuse.
In October 2007, another Chinese medicine retailer, George Zheng and his company Ichi Trade (NZ) Ltd were fined a total of $42,500 plus costs after they pleaded guilty in February 2007 to various similar charges brought under the Medicines Act 1981. George Zheng and Ichi Trade (NZ) Ltd have appealed against the penalties.
ENDS
23 April 2008
Contaminated heparin product not given to New Zealand patients
New Zealanders who were given the intravenous medication, heparin, can be reassured contaminated products found overseas have not been supplied to the New Zealand market.
Dr Stewart Jessamine, Medsafe Interim Manager says: " Medsafe has worked closely with the FDA to manage the risk of contaminated heparin products. I can reassure patients given intravenous heparin for clotting disorders in New Zealand that we are confident that no contaminated products have been distributed in this country and that the benefits of using these products far outweigh the risk of contamination".
In February 2008, the United States FDA advised Medsafe that it was investigating the safety of heparin products. This followed a number of reports in the United States of serious allergic-type reactions and very low blood pressure occurring in patients who had been give a particular brand of heparin injection during kidney dialysis or heart bypass surgery. The manufacturer of this brand of heparin had voluntarily recalled batches of their product from the United States and other countries in January.
In response to the FDA notification Medsafe contacted the distributors of injectable heparin products in New Zealand and clinicians in dialysis units to determine whether the suspect brand of heparin was available in this country, and if there had been any reports of similar side effects to those seen in the United States. This investigation found the brand in question was not used in New Zealand and that there were no problems with allergic type reactions to heparin occurring in dialysis units.
The FDA later contacted Medsafe with information that it had identified a contaminant substance within the suspect brand of heparin and provided New Zealand with the specifics of two tests that could be used to detect this substance. Medsafe subsequently contacted a New Zealand based company involved in processing the raw ingredients (these are further purified by other manufacturers of heparin products) to ensure that it was using the FDA tests on the raw ingredients it received and the processed material it exported.The company reported that some of the raw ingredients it received from China were positive for the contaminant and Medsafe reported these findings on to the FDA.
So far, testing of the finished products being supplied to patients in New Zealand indicates that these products are free from contamination.
Dr Jessamine says the requirement, introduced by the FDA and other medicines regulators, for manufacturers of heparin products to conduct testing to detect this contaminant, gives Medsafe confidence that all future imports of heparin products will be free from this problem.
Medsafe also asked the National Pharmacovigilance Centre at the University of Otago to review New Zealand's adverse reactions database for reports of allergic-type reactions to heparin and to urgently report any cases it receives to Medsafe. Clinicians working for DHBs in the field of intensive care and renal dialysis were also contacted and advised of the FDA findings and encouraged to report any unusual adverse reactions to heparin to the national centre. No reports of the serious side effects seen in the United States with heparin were identified from either the national data base or the DHBs.
The current absence of significant reports of adverse reactions to heparin in New Zealand increases Medsafe's confidence that none of the contaminated heparin has been distributed in New Zealand. Medsafe will continue to work with the New Zealand importers and distributors of heparin to ensure that supplies remain safe and free from contaminants, Dr Jessamine says.
1 February 2008
Medsafe alert of risk from anti-epileptic medicines
The Ministry of Health's medicines regulatory arm, Medsafe, is alerting health care professionals about a small increased risk of suicidal thoughts and behaviours in patients taking anti-epileptic medicines.
These medicines are used to treat epilepsy, bipolar disorder, migraine headaches and other conditions.
Medsafe's alert follows a similar statement issued by the US Food and Drug Administration (FDA) which is posted on the Medsafe website http://www.fda.gov/cder/drug/infopage/antiepileptics/default.htm
The warnings are based on studies showing that for every 1000 people taking these medicines, on average an additional two people will have suicidal thoughts or behaviours.
"We are providing doctors and health care professionals with the FDA's advice so they can inform patients and their families about the risk of suicidality as a result of taking these medicines," Medsafe's interim manager Dr Stewart Jessamine said.
"In the meantime, we advise patients to consult their doctor before making any changes to the medicines they're taking," he said.
"Alerting patients to this small additional risk means that they, and those caring for them, would be on the lookout for any unusual changes in behaviour or mood and then be able to talk to their healthcare provider."
Medsafe will be working with the companies distributing anti-epileptic medicines in New Zealand to ensure the information available to health professionals is up-to-date and includes the warning of risk of suicidality.
The FDA analysis of reports of suicidality showed that patients taking these medicines have about twice the risk of having suicidal thoughts and behaviours (0.43 per cent) compared with patients given placebo (0.22 per cent).
This means that in those groups taking these medicines an additional two (2.1) patients in every 1000 experienced suicidal thoughts than in those groups taking a harmless substitute (placebo).
Dr Jessamine said the FDA advisory will be discussed at the next meeting of the Medicines Adverse Reactions Committee (MARC) in March. MARC provides advice to the Minister of Health about medicine adverse reactions and advises on action that should be taken.
The anti-convulsant medicines approved for use in New Zealand are:
Phenobarbital (brand name Phenobarbitone)
Phenytoin sodium (brand name Dilantin)
Ethosuximide (brand name Zarontin)
Carbamazepine (brand names Tegretol, Tegretol CR,Teril)
Oxcarbazepine (brand name Trileptal)
Sodium valproate (brand name Epilim)
Tiagabine (brand name (Gabitril)
Vigabatrin (brand name Sabril)
Gabapentin (brand names Apo-Gabapentin, Arrow-Gabapentin, Neurontin, Nupentin)
Topiramate (brand names Apo-Topiramate, Topamax)
Lamotrigine (brand names Arrow-Lamotrigine, Lamictil, Lamitor DT, Logem,
Mogine, Motrig)
Levetiracetam (brand name Keppra)
Pregabalin (brand name Lyrica)
Primidone (brand name Apo-Primidone)
Note: Medsafe is the Ministry of Health's medicines regulatory arm, responsible for ensuring the appropriate level of safety and effectiveness of medicines used in New Zealand.
ENDS
18 January 2008
Children Under Two should not be given Cough and Cold Medicines
Warning: Children Under Two should not be given Cough and Cold Medicines
Please attribute to Dr Stewart Jessamine, Interim Manager Medsafe.
Cough and cold medicines should never be given to children aged less than two years of age and when given to older children extra care should be taken.
The warning issued here follows similar warnings in the United States.
An expert review, completed late last year, found that there was no evidence that cough and cold medicines reduce the symptoms of cough and colds in children.
The Medsafe and Medicines Adverse Reactions Committee (MARC) also reviewed US reports of deaths and serious side effects from accidental overdoses that occurred in children under two years.
There have been no similar reports in New Zealand, however, the National Poisons Centre has received a number of calls about overdoses of cough and cold medicines in children under two years requiring medical attention.
Based on the evidence of harm and the absence of benefit, the MARC recommended that these medicines should not be used by children under two years.
In line with action taken in the US, Medsafe is currently working towards strengthening the current warnings to indicate that these medicines must not be used in children under two years under any circumstances. Currently most labels for over-the-counter cough and cough medicines advise against use in children under two years except upon medical advice.
Medsafe advise parents:
Do not use cough and cold medicines in children aged under two years.
For children over two years:
If it is necessary to give a cough and cold product to a child, make sure that you read all labels and instructions before doing so. If the product does not contain dose information for children, then it should not be used in children.
Do not give a child a larger dose than is recommended. Do not use the product more frequently than is recommended in the labelling and instructions.
Take care not to unintentionally overdose your child. Note all the medicinal ingredients in the product, particularly if you may be giving more than one product to a child. Be aware that many products contain the same medicinal ingredient(s) and combined use could lead to overdose. Some over-the-counter medications used to control fever may also have medicinal ingredients similar to those in other cough and cold products.
Because cough and cold medications often contain multiple ingredients, it is advised not to give more than one cough and cold product to a child.
Talk to your doctor or healthcare practitioner if you have questions about the proper use of these products, dosing and administration information, or the medicinal ingredients in the products you are using.
There is no cure for the common cold. Children will usually recover from coughs and colds in time on their own. The common cold is a mild, viral infection that can be managed by rest, sufficient fluid intake and comfort measures.
In young children and babies, it is sometimes important to rule out serious illnesses (for example, pneumonia or other infections) which may present with cold-like signs and symptoms; this is especially important if symptoms persist or if the child's condition deteriorates.
If you are concerned about your child's health, the child should be brought to a healthcare practitioner for medical evaluation.
Background:
Medsafe is the Ministry of Health's drug regulatory arm. Medsafe assesses all medicines for their safety and effectiveness.
The Medicines Adverse Reactions Committee (MARC) provides advice to the Minister of Health about medicine adverse reactions and advises on action that should be taken.
Medsafe and the Medicines Adverse Reactions Committee (MARC), reviewed data on the safety and effectiveness of cough and cold medicines when used in children on 13 December 2007.
It is estimated that there are more than 100 cough and cold medicines available in New Zealand. The following active ingredients are currently used in cough and cold medicines in New Zealand:
- Expectorants (medicines that help a person cough up mucus): guaifenesin
- Mucolytics (medicines that dissolve or disperse mucus): bromhexine
- Decongestants (medicines that shrink swollen membranes in the nose): pseudoephedrine and phenylephrine
- Cough suppressants (medicines that act to suppress cough reflex): dextromethorphan and pholcodine
- Sedating antihistamines (drugs that block some of the reaction to histamines produced in the body eg clear blocked nose. These drugs frequently cause sleepiness): doxylamine, brompheniramine, chlorpheniramine, tripolidine, promethazine and diphenhydramine (NB this is also a cough suppressant)
For further information, please contact Media Advisor Michael Flyger: 0274 346 878





