Revised: 6 October 2011
Publications
Medsafe recalls two products with undeclared prescription medicines
Director-General of Health Kevin Woods today advised consumers to immediately stop taking two products for erectile dysfunction or the enhancement of sexual performance which contain undeclared prescription medicines, citing significant health risks from their use.
The warning, issued under Section 98 of the Medicines Act 1981, followed Medsafe's order for the immediate recall of all batches of the following products – Get Stiff and Maxi Mize. Medsafe is the New Zealand Medicines and Medical Devices Safety Authority. It is a business unit of the Ministry of Health and is the authority responsible for the regulation of therapeutic products in New Zealand.

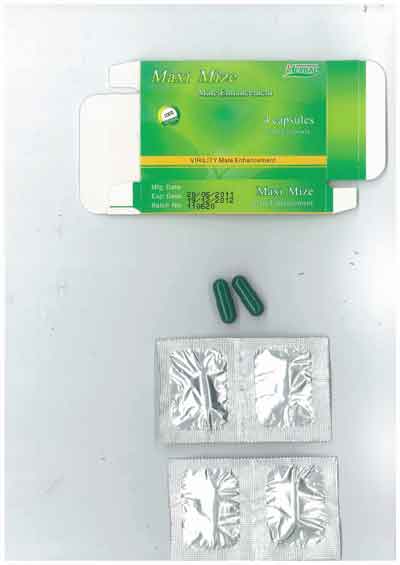
Investigations by Medsafe found that the products contained one or more of the following undeclared prescription medicines – tadalafil, vardenafil, yohimbine, hydroxyhomosildenafil and hydroxythiohomosildenafil.
“Consumers should immediately stop taking these products and seek medical advice if they have felt unwell when taking any of these products or if they are also taking other medicines,” Mr Woods said.
The two products are being promoted and sold in New Zealand by various retailers, including “adult” shops, and over the Internet as products that could enhance sexual performance or treat erectile dysfunction.
The Institute of Environmental Science and Research tested samples of the products and found them to contain significant quantities of prescription medicines.
| Product name | Undeclared medicine (dose) |
|---|---|
| Get Stiff | Vardenafil (6mg)
Yohimbine (2mg) |
| Maxi Mize | Hydroxyhomosildenafil (2mg) Hydroxythiohomosildenafil (130mg) |
Both products also contain trace amounts of tadalafil, the active ingredient
in the prescription medicine Cialis, which is used to treat erectile dysfunction.
Vardenafil is the active ingredient in the prescription medicine Levitra, which is also prescribed for erectile dysfunction. Both taladalafil and vardenafil are known to interfere with certain heart medications. Their inappropriate use can be harmful and even fatal for some people.
Hydroxyhomosildenafil and hydroxythiohomosildenafil are compounds similar in structure to sildenafil. The safety and efficacy of yohimbine hydroxyhomosildenafil and hydroxythiohomosildenafil have not been established.
"Since July 2009, Medsafe has identified 65 ostensibly herbal supplements to enhance sexual performance which have been adulterated with similar prescription medicines. The practice of adulterating this type of product with prescription medicines is common," Mr Woods cited.
"I advise consumers to treat with extreme caution products purportedly for the treatment of erectile dysfunction or to improve sexual performance offered for sale without a prescription. They should seek medical advice before using them."
Sponsors, distributors, retailers and importers are responsible for the products they sell. They are required under the Medicines Act 1981 to be aware of all the active ingredients contained in their products and to seek approval prior to selling them.
ENDS
Questions and Answers
1. What is wrong with these products?
The products have been found to be adulterated with one or more of the prescription medicines vardenafil, yohimbine, hydroxyhomosildenafil and/or hydroxythiohomosildenafil.
Vardenafil is the active ingredient in Levitra. Levitra is the only brand of vardenafil approved for sale in New Zealand to treat erectile dysfunction.
Vardenafil is known to interfere with certain heart medications and could be fatal to some individuals. Products containing vardenafil, yohimbine, hydroxyhomosildenafil, hydroxythiohomosildenafil or other similar substances should only be used on the advice of an authorised New Zealand prescriber after the benefits and risks of use have been assessed.
More information about medicines that interact with vardenafil and other precautions relating to its use can be found by accessing the Consumer Medicine Information on Medsafe’s website by typing Levitra into the search engine at: www.medsafe.govt.nz.
Hydroxyhomosildenafil and hydroxythiohomosildenafil are compounds similar in structure to sildenafil. However, as no products containing either yohimbine, hydroxyhomosildenafil or hydroxythiohomosildenafil have been approved for sale in New Zealand, their safety and efficacy have not been established.
The safety, quality and efficacy of the products in question are unknown as they have not been evaluated prior to their distribution in New Zealand through the medicines approval process.
2. If a consumer is taking one of these products, what should they do?
Consumers are being warned to immediately stop taking these products and seek medical advice from their doctor if they are taking other medicines or have felt unwell when taking the products.
Due to the way they are supplied, there is no reliable information about how many people have taken these products.
Adverse reactions to these products should be reported to the Centre for Adverse Reactions Monitoring: http://nzphvc.otago.ac.nz/report/.
Consumers can also report any concerns to Medsafe: www.medsafe.govt.nz.
3. Have these products been removed from sale?
As soon as the results from the ESR testing were received, Medsafe contacted the known distributors and retailers. They were told to remove all stock from their shelves and quarantine it, as they could not be sure about what these products contained and their sale may be in breach of the medicines legislation. They were also told to notify any other distributors and all mail order customers that they might have sold these products to, advising that the products were being recalled and that all unused products should be returned. Medsafe is continuing to investigate the matter and may take regulatory action if necessary.
4. Have the products on sale in New Zealand been tested?
Medsafe commissioned testing of the two products by ESR. The results of this testing are summarised in the following table.
| Product name | Undeclared medicine (dose) |
|---|---|
| Get Stiff | Vardenafil (6mg)
Yohimbine (2mg) |
| Maxi Mize | Hydroxyhomosildenafil (2mg)
Hydroxythiohomosildenafil (130mg) |
Information from the manufacturers and suppliers about the content of erectile
dysfunction products sold over-the-counter or over the Internet is likely
to be inadequate. It would therefore be prudent not to rely on the labels
or other promotional statements made about the ingredients in these products.
5. Where can I find more information about Levitra and its active ingredient and side effects?
Consumers seeking general information about Levitra and its active ingredient can access the Consumer Medicine information on Medsafe’s website by typing the trade name of the product into the search engine at: www.medsafe.govt.nz.
6. What about other similar products?
There are many products available from retailers and over the Internet purportedly for the treatment of erectile dysfunction or the improvement of sexual performance. Because of the illicit international trade in some of these products, products that have not been approved for sale in New Zealand may contain potentially harmful substances. Previous investigations by Medsafe have identified a number of products used to treat erectile dysfunction which have been adulterated with prescription medicines such as tadalafil and sildenafil.
The use of these products can lead to potentially serious consequences, including death. That is why Medsafe has warned against their use.
7. Important advice to traders
Under the medicines legislation, sponsors, distributors, and importers are required to obtain approval before they sell or distribute products intended for a therapeutic purpose.
Section 20 of the Medicines Act 1981 requires medicines to be approved before distribution in New Zealand. A breach of this requirement carries substantial penalties.
On conviction, the maximum penalty for an individual who sells a medicine without first having it approved through the regulatory process administered by Medsafe is $20,000 or up to six months in prison.
On conviction, the maximum penalty for a body corporate which sells a medicine without first having it approved through the regulatory process administered by Medsafe is $100,000.
The Ministry of Health takes breaches of the medicines laws very seriously, especially where patient and consumer safety is put at risk. Regulatory action will be taken as necessary to ensure compliance.
For media queries, please contact: Luz Baguioro, Media Advisor (04 496 2349, 021 802 622)





