Revised: 21 July 2006
Publications
Health warning issued by the Director-General of Health Under Section 98 Of The Medicines Act 1981 - Herbal products containing undeclared prescription medicines
Acting Director-General of Health, Debbie Chin, says people may be putting their health at risk by taking so-called herbal products which actually contain undeclared prescription medicines.
Seven different products imported from Asia, being sold as herbal medicines, have tested positive for erectile dysfunction and weight loss prescription medicines following routine investigations by the Ministry of Health Medicines Safety Authority, Medsafe.
“It is a breach of the Medicines Act 1981 to sell or supply herbal products which contain undeclared prescription medicines. There is a real potential for harm to occur when prescription medicines are used by consumers without a prescription, because of the absence of any medical supervision,” Mrs Chin says.
“If people are in doubt about the contents of any herbal product they are taking for a health condition they should seek advice from their health professional.”
Medsafe investigators located the seven products imported from Asia through a routine investigation of retail outlets. Some products containing sildenafil were being advertised as natural or herbal ‘Viagra’. Products were often being sold ‘under-the-counter’ on request and some were being supplied unlabelled in plastic bags.
Testing of the following five products showed that they contained the prescription medicine sildenafil, prescribed for the treatment of erectile dysfunction:
- 4 unlabelled Red/brown tablets marked “800mg” in a small plastic zip-lock bag
- Unlabelled bright green capsules in a small plastic zip-lock bag.
- 2 red capsules in gold foiled blister labelled Kang Da
- Orange/black capsule in a clear plastic pack with red dots.
- Cream coloured capsules (one or two) in an orange, blue and clear plastic pack
“Sildenafil is known to interfere with some heart medications and their use could be fatal to some individuals,” Mrs Chin says.
Tests on two other products, Blue/white capsules in a small plastic zip-lock bag and a pack of six capsules labelled as Qing Zhi found they contained the prescription medicine sibutramine used for the treatment of some overweight (obese) patients.
“Sibutramine can cause increased blood pressure and heart rate and cannot safely be taken by a range of people, including those with glaucoma, mental illness and severe liver or kidney problems. It should not be used in combination with other medicines such as some antidepressants and migraine treatments”, Mrs Chin says.
"Consumers should immediately stop taking these products and seek medical advice from their doctor if they are taking other medicines, have felt unwell when taking the products or if they have become unwell after they stopped taking the products."
Medsafe investigations are continuing and prosecution cases are being considered in relation to the alleged distributors. All known stocks of these herbal products have been seized.
Breaches of the medicines laws are taken very seriously, especially where patient and consumer safety is put at risk.
“We are concerned about the continuing numbers of so-called herbal medicines found to contain undeclared prescription medicines. Medsafe is actively investigating the sale and distribution of these types of products with a view to taking regulatory action against individuals or companies found to be in breach of the law,” Mrs Chin says.
Photographs of the products
ENDS
For further information please contact:
Nikki Hooper
Media Advisor
Communications
Government & Sector Relations
Ministry of Health
DDI: 470 0689
Mobile: 021 542 467
Fax: 04-496-2010
https://www.health.govt.nz
mailto: Nikki_Hooper@health.govt.nz
Questions and Answers
What are the products?
Most of the products identified as containing prescription medicines have been supplied by retailers in unlabelled plastic bags, so can only be described by their physical appearance.
Viagra is the only brand of sildenafil approved for sale in New Zealand and is used for managing erectile dysfunction. Sildenafil is known to interfere with some heart medication and could be fatal to some individuals.
Reductil is the only brand of sibutramine approved for sale in New Zealand and is used for managing overweight (obese) patients who have not been able to lose weight using a low calorie diet and exercise.
Have these products been removed from sale?
The distributors of these herbal products has been required to immediately cease supplying them. Stocks have been seized.
The products detected so far appear to have been sold through herbal product retailers. The products appear to have originated from China or from a country where Chinese language is used to label medicinal products.
If a consumer is taking one of these products what should they do?
Consumers are being warned to immediately stop taking these products and seek medical advice from their doctor if they are taking other medicines, have felt unwell when taking the products, or if they have become unwell after having stopped taking them.
There is no reliable information about how many people have taken these products.
Adverse reactions to these products or to any herbal product should be reported to the Centre for Adverse Reactions Monitoring: https://nzphvc.otago.ac.nz/report/.
Consumers can also report any concerns to Medsafe: www.medsafe.govt.nz/safety/report-a-problem.asp
Where can I find more information about Reductil and Viagra and their active ingredients and side effects?
Consumers seeking general information about Reductil and Viagra and their active ingredients sibutramine and sildenafil can access the Consumer Medication information on the Medsafe website by typing the trade name of the product into the search engine at: www.medsafe.govt.nz
Important advice to traders
Under the food and medicine legislation, sponsors, distributors and importers are required to list all active ingredients on the packaging, and to include the strength of each active ingredient.
Distributors, importers and sellers are responsible for ensuring the products they import or sell do not contain any prescription medicines. It may, for instance, be prudent to test products prior to distribution. It is illegal to sell or supply prescription medicines without the purchaser having a prescription from a registered medical practitioner.
On conviction, the maximum penalty for an individual who sells a medicine without first having it registered through the regulatory process administered by Medsafe is $20,000 or up to 6 months in prison.
On conviction, the maximum penalty for a body corporate which sells a medicine without first having it registered through the regulatory process administered by Medsafe is $100,000.
Photos of products containing sildenafil

Unlabelled Bright Green Capsules
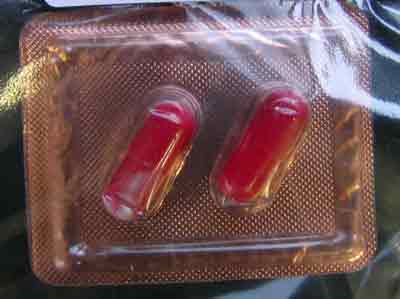

Kang Da
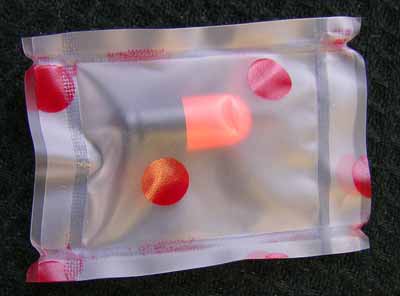
Orange/Black Capsule

Cream Coloured Capsules


Red/Brown tablets marked 800mg
Photos of products containing sibutramine

Qing Zhi Capsules

Blue/White Capsules





