Revised: 22 October 2004
Publications
Director-General's Privileged Statement Under Section 98 Of The Medicines Act 1981
Director-General of Health Dr Karen Poutasi is today warning people to stop taking four products sold as herbal remedies after investigation and testing revealed they contain undeclared prescription medicines for the treatment of erectile dysfunction.
The Ministry of Health's medicines safety authority (Medsafe) is ordering the recall of these products because they contain the undeclared prescription medicines sildenafil or tadalafil (the active substances in Viagra and Cialis respectively). The adulterated herbal products subject to this warning are not approved for supply as medicines in New Zealand and pose a safety risk to consumers if taken without medical supervision.
The products to be recalled are:
- Platinum Plus capsules
- Boyjoy tablets
- Wei Ge Wang tablets
These products contain the prescription medicine sildenafil which is known to interfere with some heart medication and could be fatal to some individuals.
Viagra is the only brand of sildenafil that is approved for sale in New Zealand and is a prescription medicine.
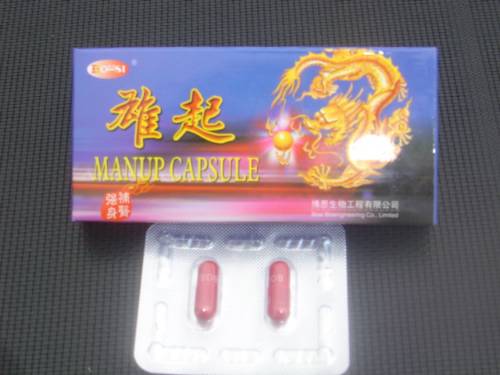
- Manup capsules
This product contains the prescription medicine tadalafil which is known to interfere with some heart medication and could be fatal to some individuals.
Cialis is the only brand of tadalafil approved for sale in New Zealand and is a prescription medicine.
Dr Poutasi says people using any of these products should immediately stop taking them and seek medical advice from their GP if they have felt unwell whilst taking them, have any concerns about their health or are taking other medications.
The products were discovered following routine testing and through information received by Medsafe.
Medsafe has required the distributors of these products to immediately cease supplying them. Where relevant, the products have been recalled from retail outlets. Further investigations are underway into the importation and supply of the products.
It is impossible to be certain herbal remedies do not contain prescription medicines without testing. Dr Poutasi says it's important consumers are cautious as there may be more herbal products at risk of containing prescription medicines or toxic substances. There are many examples in the international literature where 'herbal' products for the treatment of impotence have been found to contain prescription medicines such as sildenafil and Tadalafil.
Dr Karen Poutasi
Director-General
ENDS
Photos
Platinum Plus capsules (containing sildenafil)


Wei Ge Wang tablets (containing sildenafil)


Manup capsules (containing tadalafil)
Questions and Answers
What are the products being withdrawn?
Platinum Plus: contains the prescription medicine sildenafil. Viagra is the only brand of sildenafil approved for sale in new Zealand and is used for managing erectile dysfunction. Sildenafil is known to interfere with some heart medication and could be fatal to some individuals. Platinum Plus is labelled as a herbal supplement and lists only herbal materials as ingredients.
BoyJoy: contains the prescription medicine sildenafil. Viagra is the only brand of sildenafil approved for sale in new Zealand and is used for managing erectile dysfunction. Sildenafil is known to interfere with some heart medication and could be fatal to some individuals. BoyJoy is labeled as a Chinese herbal medicine and lists only herbal materials as ingredients. Except for the trade name, the package is labelled only in Chinese characters.
Manup: contains the prescription medicine tadalafil. Cialis is the only brand of tadalafil approved for sale in new Zealand and is used for managing erectile dysfunction. Tadalafil is known to interfere with some heart medication and could be fatal to some individuals. Manup is labelled as a Chinese herbal medicine and lists only herbal materials as ingredients.
Wei Ge Wang: contains the prescription medicine sildenafil. Viagra is the only brand of sildenafil approved for sale in new Zealand and is used for managing erectile dysfunction. Sildenafil is known to interfere with some heart medication and could be fatal to some individuals. Wei Ge Wang is labeled as a Chinese herbal medicine and lists only herbal materials as ingredients. The package is labelled only in Chinese characters.
How many people take these products in NZ?
There is no reliable information about how many people take these products but it is appears that at least 30,000 packs of Platinum Plus have been distributed.
What are the health concerns related to use of products with undeclared sildenafil and tadalafil?
The quality and therefore the safety of these medicines is uncertain. Medicines containing sildenafil and tadalafil should not be used in patients with severe hepatic impairment (liver disease), bleeding disorders (eg haemophilia), active peptic ulceration (stomach ulcers), hypotension (low blood pressure), hypertension (high blood pressure), recent history of stroke or myocardial infarction (heart attack), unstable angina (heart pain), heart failure, known hereditary degenerative retinal disorders (eye disease).
Sildenafil or tadalafil should never be used by patients on nitrate medication (used for prevention of angina) as the interaction between the two medicines can be potentially fatal.
Who is responsible for recalling the products?
Distributors and importers are required to recall the products and have been given advice on how to manage these returns. This process is overseen by Medsafe to ensure that it is performed correctly and the action is prompt.
How do consumers know what the products contain?
Usually the only way to be certain about what is present is by having the product tested. To be more confident about what they are taking, consumers should only buy products that have all the ingredients accurately listed on the product, in a language they are able to understand, or which has been accurately translated. Consumers should seek assurance from suppliers that the medicines they are purchasing are safe and do not contain other undeclared medicines.
If people are taking any of these products what should they do?
Stop taking them immediately and seek medical advice if they have felt unwell.
Can these products be sold in the interim?
No. Distributors and importers are responsible for ensuring products they import or sell do not contain any prescription medicines. Also, it is illegal to provide prescription medicines without a prescription from a registered medical practitioner.
Under food and medicine legislation sponsors / distributors / importers should list all active ingredients on packaging, write all information on packaging in the English language and include the strength of each active ingredient.
ENDS





