Revised: 2 July 2013
Safety Information
Traditional Chinese Medicines - Letter Sent to Doctors
17 January 2003
URGENT PRODUCT ALERT
File: TT05-12-60
Dear Doctor
Medsafe has been monitoring Traditional Chinese Medicines and a number of products have been tested for the presence of toxic substances and pharmaceuticals. The following products have been found to contain scheduled prescription medicines and this is being brought to your attention. As a result, these products are being withdrawn from distribution in New Zealand
The Ministry of Health will shortly be releasing a public statement on this issue. This statement will advise consumers who have been taking the following products to immediately discontinue use of the product and seek medical advice.
1. ARISTOLOCHIA
The following products have been tested and found to contain aristolochic acid:
GUAN XIN SU HE CAPSULES
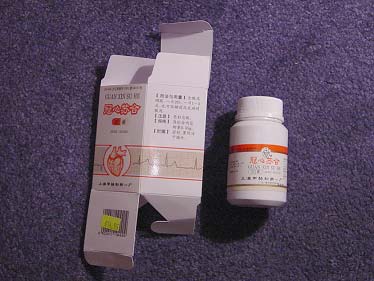
LONG DAN XIE GAN WAN SACHETS OF PILLS

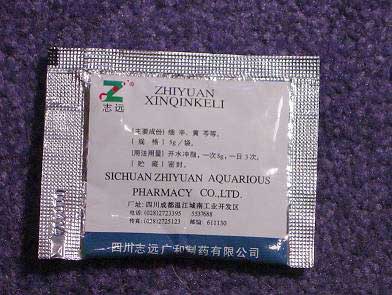
ZHIYUAN XINQINKELI SACHETS

Aristolochic acid is found in aristolochia plant species and can cause serious kidney damage and urinary tract cancer. Aristolochia species are classified as prescription medicines in New Zealand.
Medsafe is not aware of any adverse reaction reports in New Zealand associated with herbal products containing Aristolochia species, although serious adverse events have been reported in international literature.
Where patients present to you with a history of using products containing aristolochia, you should examine them for signs or symptoms suggestive of renal damage. While physical signs such as peripheral oedema warrant further investigation, renal physicians have advised that for asymptomatic patients, dipstick testing of the urine for haematuria and proteinuria, taking a serum creatinine level to allow you to calculate the patients creatinine clearance (using the formula of Cockroft and Gault*), and checking the patients' blood pressure are simple and sufficient tests to exclude significant renal damage. Where either proteinuria or haematuria is found, an MSSU should be performed to exclude urinary infection. Where significant abnormalities are found on investigation you should follow local practice with respect to referral to specialist services.
*the creatinine clearance calculator recently supplied by PHARMAC can be used to simplify this calculation.
Background information on aristolochia is enclosed with this letter.
2. SILDENAFIL
The following product has been tested and found to contain approximately 50mg Sildenafil per tablet:
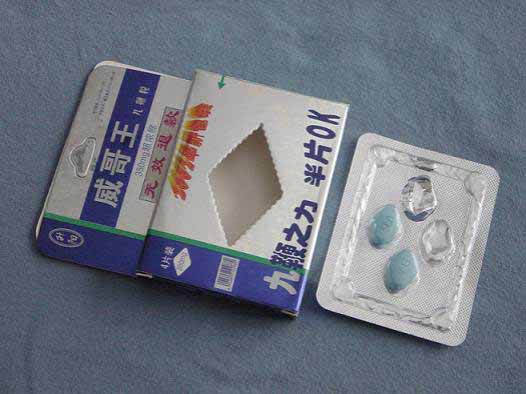
WEI GE WANG (JIU BIAN LI) TABLETS

Sildenafil is classified as a prescription medicine in New Zealand. Appropriate caution should be taken with all products from Asia marketed for the treatment of impotence.
Concomitant use of Sildenafil with nitrates is known to cause a significant and potentially life-threatening reduction in blood pressure.
3. ARSENIC
The following product has been tested and found to contain 4% arsenic:
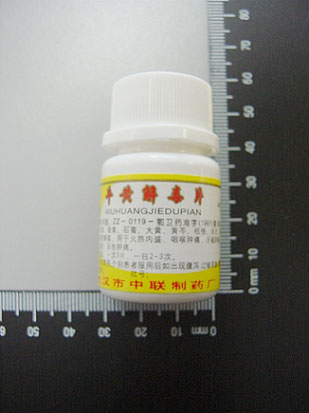
NIU HUANG JIE DU PIAN
Arsenic is classified as a prescription medicine and is a substance which can produce both acute and chronic toxicity. The dose of arsenic present in this product is likely to cause chronic toxicity.
Patients suffering from chronic arsenic toxicity can present with signs and symptoms of:
- Skin disease, such pigmentation, nail discoloration, hyperkeratosis, chronic eczematous lesions and dermatitis;
- GI disturbance, including excess salivation, "garlic" odour breath, abdominal pain, and inflammation of the oral and nasal mucosa;
- Hepatic disturbance, including jaundice and cirrhosis;
- Renal disturbance, including acute and chronic renal failure;
- Neurological deficits such as peripheral neuropathy, paraesthesia, and motor deficits; and
- Blood disorders, due to its toxic effects on the bone marrow arsenic can cause leucopaenia, thrombocytopaenia and various types of anaemia.
Patients presenting with a history of chronic use of products containing arsenic should be assessed for signs and symptoms of toxicity. Routine testing of blood count, liver and renal function may be required to exclude low levels of toxicity. Depending on your findings advice and/or referral to a specialist to manage toxicity may be necessary.
4. DICLOFENAC AND CHLORPHENIRAMINE
The following product has been tested and found to contain approximately 1mg Chlorpheniramine and 21mg Diclofenac per tablet:
GAN MAO TONG SANG JU GAN MAO PIAN TABLETS

5. DICLOFENAC, CHLORPHENIRAMINE AND PARACETAMOL
The following product has been tested and found to contain approximately 450mg Paracetamol, 3mg Chlorpheniramine and 0.05mg Diclofenac per capsule:
SHU XIAO SHANG FENG YEN QIAO JIE DU PIAN CAPSULES
The findings of the above products are an important reminder about the need for prescribers to take a complete patient history including the use of any herbal, traditional or complementary medicines and dietary supplements.
Adverse reactions suspected to be associated with such products should be reported to the Centre for Adverse Reactions Monitoring (CARM): PO Box 913, Dunedin.
Yours sincerely
Dr Stewart Jessamine
Principal Technical Specialist - Medicines Regulation and Pharmacovigilance
Medsafe
Background Information
In July 1999, two cases of nephropathy associated with the use of Chinese botanical preparations were reported in the United Kingdom (1). These preparations were shown to contain aristolochic acid. Biopsy samples showed extensive loss of cortical tubules with interstitial fibrosis.
Previously, a series of end-stage renal disease cases had been reported from Belgium associated with a weight loss treatment where one ingredient was suspected of being substituted with an aristolochia species. More than 100 patients were identified with nephropathy following the ingestion of this preparation from the same clinic from 1990-1992.
Urolethial carcinomas have been reported in some of the patients who had been diagnosed as having "Chinese herbs nephropathy" (2). Studies have shown that rodents administered aristolochic acid developed lymphoma, as well as cancers in the kidney, bladder, stomach and lung.
A number of products marketed overseas have been found to contain aristolochic acid and a number of herbs have been identified where aristolochia species have been substituted. This is because some herbs are very similar either in name or appearance or have been interchanged as part of the practise of Traditional Chinese Medicine.
The following herbs are commonly substituted with aristolochia species. These herbs may be in combinations of herbs described as mu tong or fang ji
- Akebia species
- Asarum species
- Bragantia species
- Clematis species
- Cocculus species
- Diploclisia species
- Menispernum dauricum
- Saussurea lappa
- Sinomenium species
- Stephania species
- Vladimiria souliei
Samples of the following products have been tested in Australia and found to contain aristolochic acids
- Ba Zheng San Pills (Lanzhou Taibao Medicines Factory China, Cathay Wholesale Pty Ltd)
- Chuan Xiong Cha Tiao San Pills (Lanzhou Taibao Medicines Factory China, Cathay Wholesale Pty Ltd)
- Mu Tong Akebia 5407 & Radix Stephania tetrandra (Sun Ten Pharmaceutical Co, Taiwan, Sun Ten Chinaherb Co P/L)
- Sun-Ten Hoelen & Schizandra Combination Granules (Sun Ten Pharmaceutical Co, Taiwan, Sun Ten Chinaherb Co P/L)
- Tong Ren Tang Longdan Qiegan Wan "Wetness Heat" Pill (China Beijing Tong-Ren-Tang Australian Company Pty Ltd)
- Xiao Feng San Pills (Lanzhou Taibao Medicines Factory China, Cathay Wholesale Pty Ltd)
- Xiao Qing Long Wan Pills (Lanzhou Taibao Medicines Factory China, Cathay Wholesale Pty Ltd)
- Xiao Qing Long Wan Pills (Shen Neng Pty Ltd)
- Xin Yi San Pills (Lanzhou Taibao Medicines Factory China, Cathay Wholesale Pty Ltd)
- Xuan Bi Tang Pills (Lanzhou Taibao Medicines Factory China, Cathay Wholesale Pty Ltd)
The following products have mu tong and fang ji as declared ingredients and may therefore contain aristolochia
- Ba Zheng Wan
- Chun Yang Zheng Ji Wan
- Da Huang Qing Wei Wan
- Dang Gui Si Ni Wan
- Dao Chi Wan
- Dieda Wan
- Fu Ke Fen Quing Wan
- Ji Sheng Ju He Wan
- Kat Kit Wan
- Quell Fire
- Shi Xiang Fan Shen Wan
- Xin Yi Wan
References:
- Lord GM et al (1999) "Nephropathy caused by Chinese herbs in the UK". Lancet 354:481-482
- Nortier JL et al (2000) "Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi)". New England Journal of Medicine 342:1686-1692.
Issued by Medsafe on 17 January 2003





