Published: 22 February 2005
Safety Information
Product Alert - Monofeme and Monofeme 28
22 February 2005
Important message for women taking Monofeme or Monofeme 28 Oral Contraceptive
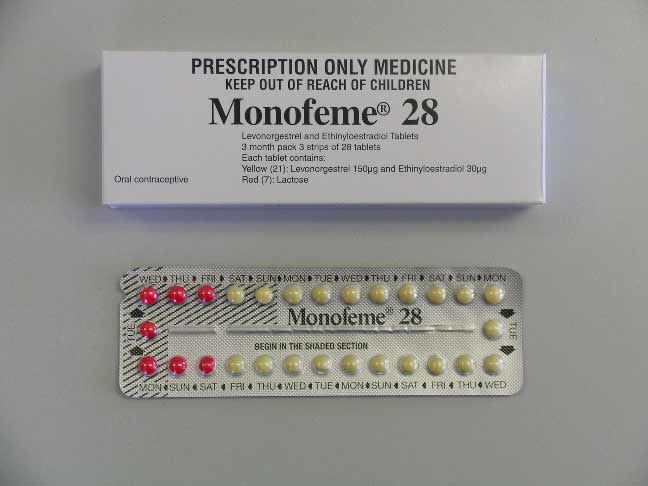
Wyeth has recently updated the packaging of the oral contraceptive, Monofeme 28. It has been repackaged and the product name has changed to Monofeme (the '28' has been removed). Until recently, both Monofeme (new pack) and Monofeme 28 have been available.
Wyeth is asking all women taking Monofeme 28 or Monofeme to check their current packs. Further advice on identifying the different packs may be obtained from a pharmacy.
Women who have started on Monofeme (new pack) must NOT switch back to Monofeme 28 (old pack). This is to ensure their contraceptive protection is maintained. If this switch back to Monofeme 28 has occurred, advice should be sought from a medical practitioner without delay.
Women who have started on Monofeme (new pack) and still have Monofeme 28 (old pack) should return the unused packs of Monofeme 28 (old pack) ONLY to their pharmacist for replacement and advice.
Women who only have packs of Monofeme 28 (old pack) and have not taken Monofeme (new pack) need take no further action now other than to continue taking the Monofeme 28 as normal. Changeover to Monofeme (new pack) will occur when the next prescription is dispensed.
It is important to note that there is no issue with the quality, safety or efficacy of either product. Women should continue their current cycle of oral contraceptive.
This action has been taken after consultation with Medsafe.
Further information is available from Wyeth NZ by calling freephone 0800 447 400 between 9am and 7pm.
For Health professionals
Information has been provided by Wyeth directly to pharmacies and prescribers regarding the return of Monofeme 28 packs and advice for consumers.
Photos
Monofeme
Monofeme 28