Published: 23 July 2020
Updated: 2 March 2021
Safety Information
Alert
Yes! Cassette Pregnancy Test Kit (also known as Smith BioMed Rapid Pregnancy Test Kit) – Reports of false positives presenting as faint lines and inconclusive results
Update Information
2 March 20211
Smith BioMed is recalling all remaining batches of the Yes! Cassette Pregnancy tests (also known as Smith BioMed Rapid Pregnancy Test Kit). See the table below for the affected batch numbers.
The recall is due to continued issues with the test kits, with false positives and inconclusive results being reported. Two batches were recalled in September 2020, and this March 2021 recall is for the remaining three batches
|
February 2021 recall – Affected batch numbers and expiry dates |
|
|
September 2020 recall – Affected batch numbers and expiry dates |
|
|
These are supplied in kits of 40 tests, 20 kits per carton. |
SmithBioMed will be issuing a recall notice and will be in contact with
users of these test kits to replace the affected stock with
PHARMAC-funded pregnancy tests:
- David Step Pregnancy Cassette Test. 25mlU/. Supplier/Pharmacode: DAVCASS /2602318.
Please note that pregnancy test kits are only intended to provide indicative results. If the result obtained is inconclusive or different to that expected, then instructions for use recommend retesting. Any remaining doubt can be removed by doing a blood test.
If you require any information about this recall action, please contact Smith BioMed on:
- Phone: 0508 246 633
- Email: info@smithbiomed.com
Update Information
03 September 2020
Smith BioMed is recalling two batches of the Yes! Cassette Pregnancy Test kits. The recalled batches have higher than expected reports of false positives.
Smith BioMed will issue a recall notice to users of these test kits and arrange for replacement batches to be distributed.
| Affected batch numbers and expiry dates |
|
| These are supplied in kits of 40 tests, 20 kits per carton. |
If you require any information about this recall action, please contact
Smith BioMed on:
- Phone: 0508 246 633
- Email: info@smithbiomed.com
This recall does not apply to other batches. Medsafe encourages healthcare professionals to continue using batches not affected by this recall, and to continue reporting if there are any issues with other batches.
Medsafe will continue to monitor this issue and will produce updated advice for health care professionals as necessary.
Original Communication
24 July 2020
Medsafe has received several reports from clinicians, DHBs, clinics and community providers about the Yes! Cassette Pregnancy Test Kits (also known as the Smith BioMed Rapid Pregnancy Test), shown in Figure 1 below. The reports describe false positive test results and inconclusive results. These present as faint lines.
Medsafe is asking health care professionals to report any issues experienced with these test kits, to assist with our decision making.
Products affected
Additional information
Regulator actions
Reporting
Products affected
| Product name | Sponsor |
|---|---|
| Yes! Cassette Pregnancy Test Kit All Batch numbers: |
Smith BioMed New Zealand |
Additional information
These tests are used as an early detection test for pregnancy. The test is accurate if used 7 days after the next period is due. Hormone levels in women vary and some women may not have detectable levels of hormone on the first day they test for it.
Yes! Cassette Pregnancy Test Kit information leaflet
PHARMAC funds this pregnancy test kit. However, on the Pharmaceutical Schedule, the test kit is called the Smith BioMed Rapid Pregnancy Test (2544873).
Figure 1: Image of the test kit box (a) and test kit (b)
(a)  |
(b) 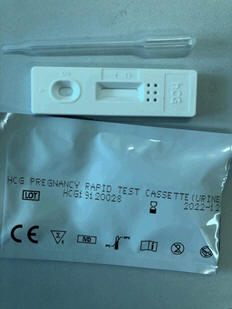
|
Regulator actions
Medsafe will continue to monitor this issue and will produce updated advice for health care professionals as necessary.
Reporting
Health care professionals are encouraged to report issues with this product to Medsafe. Please include in your report:
- a description of the issue
- the total number of affected tests
- batch numbers
- any other relevant information.
If you have questions about this monitoring communication, please email: devices@health.govt.nz





