Revised: 28 May 2013
Consultations
Review of Administrative Processes Associated with the Evaluation and Classification of Medicines
30 April 2009
To:
Product sponsors
Applicants for consent to distribute new and changed medicines and related
products
Regulatory affairs consultants
The purpose of this letter is to:
- signal planned changes to administrative processes that support the consent processes for new and changed medicines/related products and the classification of medicines;
- outline implementation and transition arrangements for the change process; and
- provide a contact point for any queries about, or feedback on, the proposed changes
Background
Medsafe has undertaken a review of its administrative processes in order to identify opportunities to improve efficiency, provide better clarity and more useful information in standard letters, and ensure that its processes are appropriately aligned with the requirements of the Medicines Act 1981. Change proposals identified early in the review were discussed at an industry seminar attended by SMI and RMI members in late 2008. The next meeting in this series (to be held on 6 May 2009) will provide a further opportunity for discussion.
Changes arising from the review
- Introduction of invoicing for the payment of fees
This change will mean that companies are no longer required to send money with an application or notification. Following receipt of an application or notification, an invoice (in the name of the sponsor) will be sent to the applicant with an acknowledgement letter. Payment will be requested within 7 days and will be required to validate the application/notification. Reminders will be sent in relation to unpaid invoices. Applications and notifications will, however, be invalid if payment has not been received by the 20th of the month following the month in which the invoice was issued.
The move to invoicing is expected to benefit both the Ministry and applicants, as both parties find the current system cumbersome and inefficient. The invoicing system will be underpinned by an electronic interface between Medsafe's SMARTI system and the Ministry's financial management system that will facilitate the matching of payments with invoice numbers relating to particular applications/notifications.
Transition arrangements will be in place to ensure that:- payments sent prior to the changeover are able to be processed under the old system; and
- for a limited time, up-front payments sent in error will be processed, with the applicant receiving a reminder about the new invoicing system.
- New style template letters
New template letters have been developed for process-related communications such as acknowledgement of applications, requests for further information, and advice about the next steps in a process. The new letters have been designed to improve the clarity and utility of the information provided and also reflect the move to invoicing. For example:- An acknowledgement letter will include the decision on any request for a fee waiver or for priority assessment, rather than this occurring later and requiring a separate communication; and
- The letter acknowledging a self-assessable change notification will advise whether the file has been selected for audit and, if so, the outcome of the audit.
- Referral of Changed Medicine Notifications under Section 24(5)
Medsafe will refer changed medicine notifications to the Minister under Section 24(5)(b) of the Act if evaluation queries cannot be resolved within 90 days of receipt of a valid CMN. This change will clarify the legal situation in relation to changed medicine notifications. Following referral under section 24(5), the company will not be able to lawfully implement the change(s) until the Minister has notified consent in the Gazette.
The change will NOT result in delays in dealing with responses when they are supplied by companies. - Referral to the Medicines Assessment Advisory Committee
A change to the referral process for new medicines requiring consideration by the Medicines Assessment Advisory Committee (MAAC) has already been implemented and advised to companies. The new process means that the evaluation of products and the response to queries raised during the evaluation will have been completed before the MAAC consideration. This change is expected to shorten, rather than lengthen, evaluation times and is likely to mean that fewer medicines will need to be referred to the committee. A flow diagram describing the new process is attached as Appendix I. - Processing of updated Drug Master Files
When an updated DMF is received directly from an active pharmaceutical ingredient manufacturer, Medsafe will check whether the manufacturer has identified the changes that have been made or stated that there are no material changes. If neither of these has occurred, Medsafe will write to sponsors who use that ingredient to:- advise that a DMF update has been received;
- ask the sponsor to ascertain the nature of the changes and advise Medsafe whether or not they are material; and
- submit a CMN if the DMF changes are material.
Next steps
The target implementation date for the changes described above is 1 July 2009 .
Queries about, or comments on, the changes described above should be sent to
Susan Martindale
Principal Advisor Regulation
Medsafe
PO Box 5013
WELLINGTON
Email susan_martindale@health.govt.nz
by Friday 29 May 2009.
Comment on the feedback received and any resulting adjustments to the new processes will be provided through the Medsafe web update on 17th June 2009. The implementation date will also be confirmed at this time.
Yours sincerely
Susan Martindale
Principal Advisor Regulation
Guidance on the processing of applications requiring Medicine Assessment Advisory Committee (MAAC) review.
Changes have been made to the handling of medicine applications requiring MAAC assessment. These changes have been made to more closely match the process to legislative requirements. An overview of the new process is described in diagram 1.
Diagram 1: Process for the review of application requiring MAAC referral.
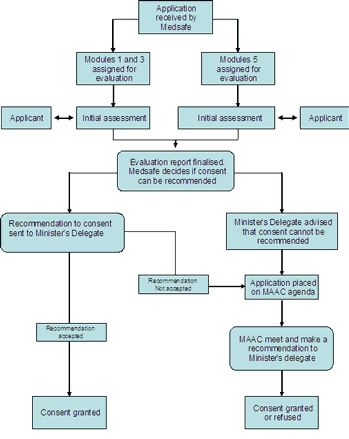
The process consists of the following 6 steps:
- A new medicine application is received by Medsafe or a notification is referred to the Minister under Section 24(5) of the Medicines Act 1981.
- The application is assigned to appropriate evaluators for the assessment of the clinical and pharmaceutical chemistry data.
- Evaluation is undertaken, issues resolved if possible, and an evaluation report prepared.
- Medsafe either makes a recommendation to the Minister's delegate to grant consent OR arranges referral to MAAC for further advice.
- The MAAC considers applications referred to it and makes a recommendation to the Minister's delegate on whether or not consent should be granted.
- The Minister's delegate makes a decision on whether to grant or refuse to grant consent.
Key features of the process
- The pharmaceutical chemistry and clinical assessment of a New Medicine Application or Changed Medicine Notification referred under Section 24(5) will commence as soon as feasible after an application is received by Medsafe. The timing of evaluations is no longer linked to MAAC meeting dates.
- Once initial evaluation is completed, further information will be requested from the applicant to resolve any issues that have arisen during evaluation. A copy of the evaluation report will be provided to the applicant.
- Up to two requests for information may be made in an attempt to resolve any outstanding clinical or pharmaceutical chemistry issues before the evaluation report is finalised.
- Based on the finalised evaluation report, Medsafe will either make a recommendation to the Minister to grant consent or advise the Minister that consent cannot be recommended.
- In deciding whether to recommend that consent is granted, Medsafe will take into account recommendations made by the evaluator and other factors such as the nature of the medicine, the quality of the data submitted, the conclusions reached by the evaluator and whether the medicine has been approved by other reputable regulatory authorities.
- Where Medsafe's recommendation is to grant consent and the Minister accepts this recommendation, the consent process will proceed without the application being considered by the MAAC.
- Where Medsafe is not able to recommend that consent is granted, the application will be considered by the MAAC.
- The MAAC meeting agenda will be finalised one month prior to the date of the meeting. The applicant will be notified that their application has been placed on the MAAC agenda.
- The MAAC will make a recommendation to the Minister to grant or refuse to grant consent. The final decision rests with the Minister and is appealable.
- In the case of existing, outstanding applications, if the applicant can provide evidence that the product in question has been approved by other recognised authorities then Medsafe may not need to refer the applications back to the MAAC





