Published: 7 March 2019
Publications
Bullous pemphigoid – A blistering problem
Prescriber Update 40(1): 19–21
March 2019
Introduction
Dipeptidyl peptidase-4 (DPP-4) inhibitors (also known as ‘gliptins’), used to treat type 2 diabetes mellitus, have recently been associated with bullous pemphigoid1.
What is bullous pemphigoid?
Bullous pemphigoid is an autoimmune disease that results in subepidermal blistering1. Autoantibodies and activated T lymphocytes target proteins in the basement membrane of the epidermis, triggering the inflammatory process, which leads to blister formation2.
Most cases of bullous pemphigoid occur in individuals aged over 60 years3. It usually presents with severe itching and large (1–3 cm) fluid-filled blisters, called bullae (Figure 1). The bullae eventually burst leaving moist erosions and crusts that resolve without scarring. Mucous membranes may also be involved2,4,5.
Bullous pemphigoid is usually a self-limiting disease with a clinical course that may last from months to years4. However, it can be a serious and potentially fatal disease, particularly when lesions are widespread or resistant to treatment2,6.
Figure 1: Bullous pemphigoid images
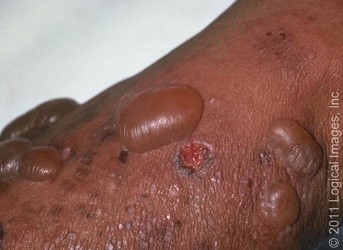
a: Multiple tense bullae on skin with one eroded blister base
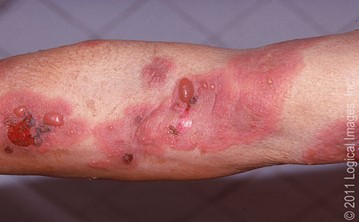
b: Arm skin with tense bullae arising on urticarial plaques and
eroded blister bases
Source: Leiferman KM. 2018. Clinical features and diagnosis of bullous pemphigoid and mucous membrane pemphigoid. In: UpToDate. 30 November 2018. URL: www.uptodate.com/contents/clinical-features-and-diagnosis-of-bullous-pemphigoid-and-mucous-membrane-pemphigoid (accessed 18 January 2019). Reproduced with permission from visualdx.com.
What are the causes of bullous pemphigoid?
The risk of bullous pemphigoid is increased with2,4:
- advanced age
- certain HLA associations, indicating a genetic predisposition
- exposure to some medicines (see the following section)
- comorbidities such as neurological disease (eg, stroke, dementia, Parkinson’s disease), psoriasis, cancer, skin infection.
What medicines are associated with bullous pemphigoid?
Penicillamine and furosemide are most frequently implicated from case reports of drug-induced bullous pemphigoid7. Cases associated with captopril, penicillin and its derivatives, sulfasalazine, and topical fluorouracil have also been reported internationally. Case-control studies have found a significant association between bullous pemphigoid and neuroleptics, loop diuretics, and spironolactone7.
Recently, DPP-4 inhibitors have been associated with bullous pemphigoid1,8. Evidence for this association was initially based on case reports and national pharmacovigilance database analyses, and now includes controlled observational studies8. The pathomechanism underlying the association between DPP-4 inhibitors and bullous pemphigoid is not yet fully understood.
Vildagliptin, sitagliptin, and saxagliptin are the approved DPP-4 inhibitors in New Zealand. Bullous pemphigoid is listed as an adverse event derived from post-marketing experience in the vildagliptin9,10 and sitagliptin data sheets11,12.
New Zealand case reports
In New Zealand, between 1965 and 2018, the Centre for Adverse Reactions Monitoring (CARM) received 20 reports of pemphigoid reactions suspected to be caused by a medicine. One of these reports was associated with D-penicillamine (CARM ID number: 012003), one with furosemide (001113) and three with penicillin derivatives (000929, 098771, 114683). There were no reports of pemphigoid reactions associated with DPP-4 inhibitors.
What is the treatment for bullous pemphigoid?
If a medicine is suspected to be causing the bullous pemphigoid, discontinue the medicine and consider referral to a dermatologist. First-line treatment of bullous pemphigoid usually involves topical or systemic corticosteroids and supportive care. However, immunosuppressant therapy may be required6.
References
- Kridin K and Ludwig RJ. 2018. The growing incidence of bullous pemphigoid: overview and potential explanations. Frontiers in Medicine 5: 220. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC6109638/ (accessed 1 February 2019).
- Oakley A. 2016. Bullous Pemphigoid. In: DermNet NZ January 2016. URL: www.dermnetnz.org/topics/bullous-pemphigoid/ (accessed 18 January 2019).
- Leiferman KM. 2019. Epidemiology and pathogenesis of bullous pemphigoid and mucous membrane pemphigoid. In: UpToDate. 15 January 2019. URL: www.uptodate.com/contents/epidemiology-and-pathogenesis-of-bullous-pemphigoid-and-mucous-membrane-pemphigoid/ (accessed 18 January 2019).
- Venning VA, Taghipour K, Mohd Mustapa MF, et al. 2012. British Association of Dermatologists’ guidelines for the management of bullous pemphigoid 2012. British Journal of Dermatology 167: 1200–14. URL: www.bad.org.uk/shared/get-file.ashx?id=47&itemtype=document (accessed 18 January 2019).
- Leiferman KM. 2018. Clinical features and diagnosis of bullous pemphigoid and mucous membrane pemphigoid. In: UpToDate 30 November 2018. URL: www.uptodate.com/contents/clinical-features-and-diagnosis-of-bullous-pemphigoid-and-mucous-membrane-pemphigoid/ (accessed 18 January 2019).
- Murrell DF and Ramirez-Quizon M. 2018. Management and prognosis of bullous pemphigoid. In: UpToDate 14 November 2018. URL: www.uptodate.com/contents/management-and-prognosis-of-bullous-pemphigoid/ (accessed 18 January 2019).
- Samel A and Chu C-Y. 2018. Drug eruptions. In: UpToDate 10 April 2018. URL: www.uptodate.com/contents/drug-eruptions (accessed 18 January 2019).
- Gaudin O, Seta V, Alexandre M, et al. 2018. Gliptin accountability in mucous membrane pemphigoid induction in 24 out of 313 patients. Frontiers in Immunology 9: 1030. URL: www.ncbi.nlm.nih.gov/pmc/articles/PMC5976795/ (accessed 18 January 2019).
- Novartis New Zealand Limited. 2017. Galvus 50 mg tablet New Zealand Data Sheet 30 January 2017. URL: www.medsafe.govt.nz/profs/Datasheet/g/galvustab.pdf (accessed 1 February 2019).
- Novartis New Zealand Limited. 2018. Galvumet 50 mg/850 mg, 50 mg/1000 mg tablets New Zealand Data Sheet 18 August 2018. URL: www.medsafe.govt.nz/profs/Datasheet/g/galvusmettab.pdf (accessed 1 February 2019).
- Merck Sharp & Dohme (New Zealand) Limited. 2018. Januvia 25 mg, 50 mg, 100 mg film-coated tablets New Zealand Data Sheet 5 April 2018. URL: www.medsafe.govt.nz/profs/Datasheet/j/Januviatab.pdf (accessed 1 February 2019).
- Merck Sharp & Dohme (New Zealand) Limited. 2018. Janumet 50/50 mg, 50/850 mg, 50/1000 mg film-coated tablets New Zealand Data Sheet 5 April 2018. URL: www.medsafe.govt.nz/profs/Datasheet/j/Janumettab.pdf (accessed 1 February 2019).





