Updated: 10 February 2025
Medicines
What to do in the case of a potential out of stock
Do you have a potential out of stock (OOS) issue that may require
regulatory action?
What information does Medsafe require?
How to notify Medsafe
How do OOS issues occur?
Regulatory actions required to resolve an OOS issue
How to ensure that OOS issues are resolved efficiently
Resolving OOS issues for controlled drugs
Do you have a potential out of stock (OOS) issue that may require regulatory action?
Medsafe should be informed of an OOS issue that may require regulatory action as soon as possible. In situations where regulatory action is required to resolve a potential OOS event, clear, informative communication with Medsafe can help minimise disruption to Medsafe workflows and may allow for a faster, easier resolution.
Medsafe (which includes Medicines Control) works in collaboration with PHARMAC (if the product is listed on the Pharmaceutical Schedule) to assist with the resolution of OOS issues, to ensure continued availability of essential medicines for New Zealand patients.
What information does Medsafe require?
Please provide the following information:
- What is the reason for the OOS issue?
- How much stock is there in the supply chain (include supply held by the sponsor, wholesalers and retailers)?
- How long will the current stock last (include total volume and weekly/monthly usage in response)?
- What steps have been taken to resolve the OOS issue?
- What action do you propose to resolve and/or mitigate the impact of the OOS issue?
- What clinically equivalent or alternate products are:
- approved in NZ, available and PHARMAC funded;
- approved in NZ, available but not PHARMAC funded;
- approved in NZ but not currently available;
- not approved for use in NZ but able to be obtained to meet clinical need.
- Is any regulatory activity required for either a proposed alternate product, or to enable supply of the current product?
- Will any regulatory activity require urgent assessment in order to prevent
an OOS situation?
- If so, Medsafe requires a priority support letter (eg, from PHARMAC) that confirms the proposed course of action best mitigates the risk of disruption to patients. If the medicine is PHARMAC funded, PHARMAC must be informed and support the proposed course of action.
All supporting information relating to a potential OOS issue that requires regulatory action should be included in the cover letter that accompanies the application.
Further information is available on the definitions of registration situations and Medsafe’s policy for changing registration situations.
How to notify Medsafe
Sponsors should notify Medsafe of a potential OOS issue associated with a potential quality (including GMP) issue with a medicine via recalls@health.govt.nz.
Sponsors should notify Medsafe of a potential OOS issue where regulatory action is required via medsafeapplications@health.govt.nz.
How do OOS issues occur?
A product recall could cause an OOS issue that may require regulatory action. Refer to the recalls code for further information on how a product recall is decided on and executed.
Other factors that may create an impending OOS issue include:
- manufacturing delays
- product recalls
- transport-related temperature deviations
- stability issues identified during stability monitoring
- product received in NZ is not as per NZ approved product
- discontinuation of active pharmaceutical ingredient (API)/medicine product
- worldwide shortage of API/medicine product
- delays as a result of import licence requirements
- Good Manufacturing Practice (GMP) issues.
Regulatory actions required to resolve an OOS issue
The sponsor should determine whether any of the following regulatory actions are required to address the potential OOS issue:
- pre-market assessment and approval of a new medicine via the full or abbreviated New Medicine Application (NMA) regulatory pathway; or
- pre-market assessment and approval of a changed medicine via the Changed Medicine Notification (CMN) or CMN section 24(5) regulatory pathway.
Indicative evaluation timeframes for each regulatory provision above can be found at www.medsafe.govt.nz/regulatory/EvaluationTimeframesAndRegistrationSituation.asp. It is pertinent to note that well formatted, comprehensive, high quality applications are likely to minimise delays to evaluation timeframes.
If time does not permit for the standard indicative timelines of regulatory activities to prevent a potential OOS issue, a request for priority assessment can be made to Medsafe at the time the application is submitted. Medsafe offers priority assessment under three criteria, further outlined in Section 3.5 of Part 2 of the Current Guidelines on the Regulation of Therapeutic Products in New Zealand. These criteria apply to NMAs only (this includes CMNs referred under Section 24(5) of the Medicines Act 1981).
Note that CMNs are not eligible for priority assessment. However, Medsafe may adjust its workflows for CMNs in cases where an expedited assessment would ensure patient treatment with critical medicines is not interrupted.
Sponsors should note that urgent assessment can consume significant resource and have a disruptive downstream effect on Medsafe’s workflows. Priority designation is always dependant on available Medsafe resource.
In addition, post-market action on medicines currently in the market place may be required in some instances.
How to ensure that OOS issues are resolved efficiently
There are a number of steps that sponsors can take to ensure that any regulatory submissions to resolve an OOS issue are processed efficiently. These steps are listed below.
- Along with supporting information, a clear statement that the submission relates to an OOS should be included in the cover letter.
- Invoices should be paid promptly. In urgent cases, proof of payment may be forwarded to Medsafe to allow evaluation to begin immediately.
- Requests for information for an OOS application should anticipated and their response should be prioritised as necessary.
Resolving OOS issues for controlled drugs
See ‘Licence to Import Controlled Drugs’ workflow.
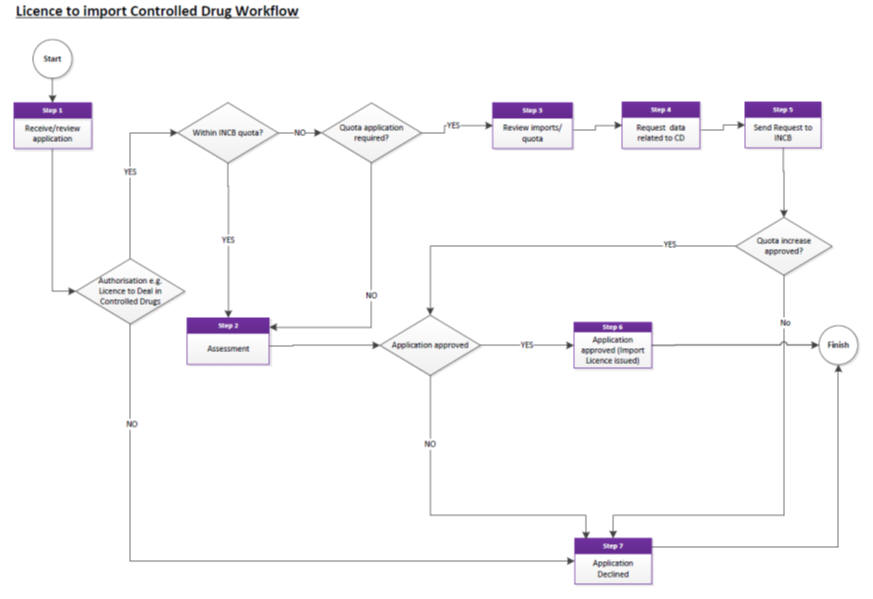
Applicants should allow up to 30 working days for the processing of import applications.





