Published: 27 November 2020
Archived: 21 August 2024
About Medsafe
COVID-19 Archive
Vaccine Evaluation and Approval Process
- Communicating with pharmaceutical companies
- Rolling applications
- Abbreviated evaluation pathway
- Priority review
- Seeking expert advice
- Decision on approval
- Post-approval safety monitoring
- Further information
Medsafe evaluates applications for all new medicines, including vaccines, to ensure that they comply with international standards and local requirements for quality, safety and efficacy. Only if the medicine meets these standards will Medsafe recommend approval for use in New Zealand. More information about the medicines approval process.
Due to the ongoing pandemic, there is an urgent clinical need for safe and effective vaccines to protect New Zealanders against COVID-19. To meet this need, Medsafe will be streamlining assessment processes and prioritising the evaluation of COVID-19 vaccines over other medicines to obtain a vaccine more quickly – as described below. However, we will not compromise on the integrity of the process or on the safety of the vaccine.
Communicating with pharmaceutical companies
Medsafe is in regular communication with pharmaceutical companies to gather information on the COVID-19 vaccines under development and to discuss their plans for submitting vaccine applications. This regular communication means that we can prepare for incoming vaccine applications and ensures that companies submit applications in a consistent way.
Rolling applications
Medsafe is allowing pharmaceutical companies to make rolling applications for COVID-19 vaccines.
Normally a standard New Medicine Application contains all the required information in a single package (dossier). Medsafe’s evaluation starts once the full dossier has been submitted (see Figure 1). More information about New Medicine Application evaluation timeframes.
A rolling application allows companies to submit different sections of the dossier to Medsafe as soon as the data becomes available (Figure 1). For example, companies may provide data about the manufacture of their vaccine to Medsafe before the initial clinical trial results are available. We will evaluate each section as it is received, meaning that we may be able to make a final recommendation much sooner than with the standard application process.
Figure 1: The standard new medicine application and evaluation process and the COVID-19 vaccine rolling application and evaluation process
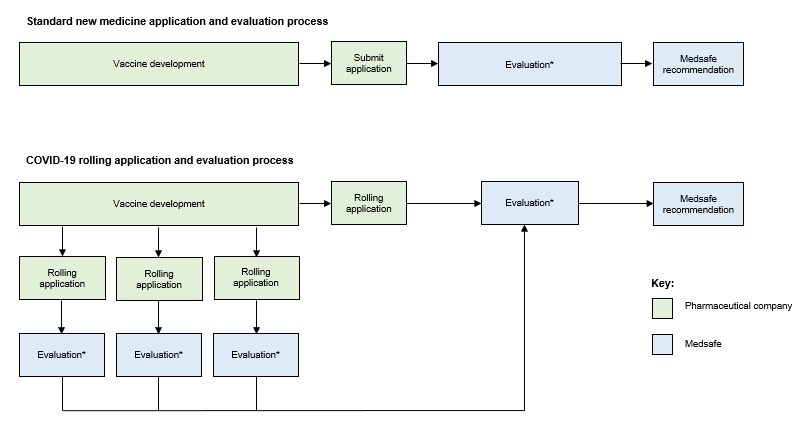
* Evaluation includes the time spent by Medsafe reviewing data and the time taken by companies responding to Medsafe’s requests for further information. For COVID-19 vaccines, evaluation may also include requests for advice from the COVID-19 Vaccine Advisory Group and referral to the Medicines Assessment Advisory Committee (see below).
Abbreviated evaluation pathway
Medsafe has an abbreviated evaluation pathway in which review of overseas regulatory evaluation reports forms the basis of the evaluation. Companies may choose to use the abbreviated evaluation pathway for their COVID-19 vaccines.
The abbreviated evaluation pathway shortens evaluation times but depends on the availability of overseas evaluation reports. If a comparable rolling application process is followed in both New Zealand and another country, companies may be able to use a rolling abbreviated pathway.
Medsafe is also working closely with other medicines regulators and is involved in international collaborative initiatives to ensure a consistent approach to COVID-19 vaccine evaluation.
Priority review
Medicine applications that have been accepted for priority review are processed earlier than normal applications.
So long as an urgent clinical need for safe and effective COVID-19 vaccines remains, these applications will be granted priority review status. This means that we can make recommendations about COVID-19 vaccines sooner than if they were evaluated under the normal process.
Seeking expert advice
Medsafe staff have the knowledge and experience needed to evaluate the quality, safety and efficacy of a broad range of medicine types. However, COVID-19 vaccines have been rapidly developed and many use novel technologies not used in any other approved vaccines. To ensure that we make scientifically sound decisions, we will seek advice from the COVID-19 Vaccine Advisory Group and may also refer vaccines to the Medicines Assessment Advisory Committee.
COVID-19 Vaccine Advisory Group
This advisory group is composed of experts in a range of relevant fields who will advise Medsafe on specific questions raised during the COVID-19 vaccine application evaluations. This advice will be provided in parallel with Medsafe’s evaluation and will not delay the application process.
Medicines Assessment Advisory Committee (MAAC)
Medsafe can also refer COVID-19 vaccines to the MAAC for a final recommendation about approval. The MAAC will consider any COVID-19 vaccines referred to them under urgency. More information on the MAAC.
Decision on approval
Once we have completed the vaccine evaluation process, and following possible consideration by the MAAC, we will make a recommendation about vaccine approval. Only if the vaccine meets internationally agreed criteria for safety and efficacy will we recommend approval for use (consent).
If consent can be granted, this will either be full consent under section 20 of the Medicines Act 1981 or provisional consent under section 23. We expect that most vaccines will be granted provisional consent because data to support the longer-term safety and efficacy of COVID-19 vaccines is not yet available.
Provisional consent allows conditions to be imposed on the vaccine, restricting its use by healthcare professionals according to the data available at the time of approval. Provisional consent was included in the Medicines Act to allow New Zealand patients to have early access to medicines with a significant unmet clinical need.
Post-approval safety monitoring
Medsafe uses a variety of methods to collect information on the safety and quality of medicines and vaccines after they have been approved.
- Information about Medsafe’s usual medicine safety monitoring process
- Information about Medsafe’s usual vaccine safety monitoring process
- COVID-19 Vaccine Safety Monitoring Process





