Published: June 2012
Publications
NSAIDs can SCAR (Severe Cutaneous Adverse Reaction)
Prescriber Update 33(2): 11-12
June 2012
NSAIDs are medicines widely used for the relief of pain and inflammation and are commonly purchased over-the-counter in addition to being prescribed. Non-steroidal anti-inflammatory drugs (NSAIDs) can cause severe cutaneous adverse reactions (SCARs) in rare cases.
SCARs include bullous eruptions, erythema multiforme, epidermal necrolysis, toxic epidermal necrolysis and Stevens Johnson syndrome. The Centre for Adverse Reactions Monitoring (CARM) has received a number of reports of SCARs associated with NSAIDs (Figure 1).
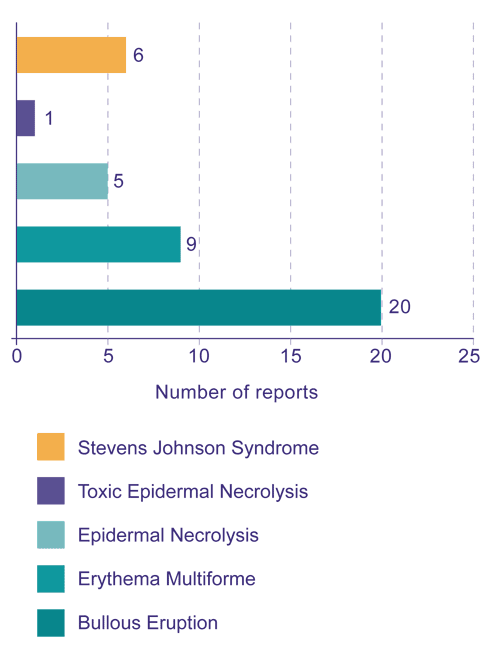
Figure 1: CARM reports of severe cutaneous adverse reactions associated with NSAIDs
SCARs may cause permanent sequelae such as disfigurement, blindness and death. Importantly, these reactions may occur without warning.
The overall risk of SCARs associated with the use of NSAIDs is extremely low. The highest reported incidence is with celecoxib at six cases per 1 million person-years1. In New Zealand, the NSAIDs most commonly reported to cause SCARs are piroxicam, naproxen, diclofenac, celecoxib and ibuprofen (Figure 2).
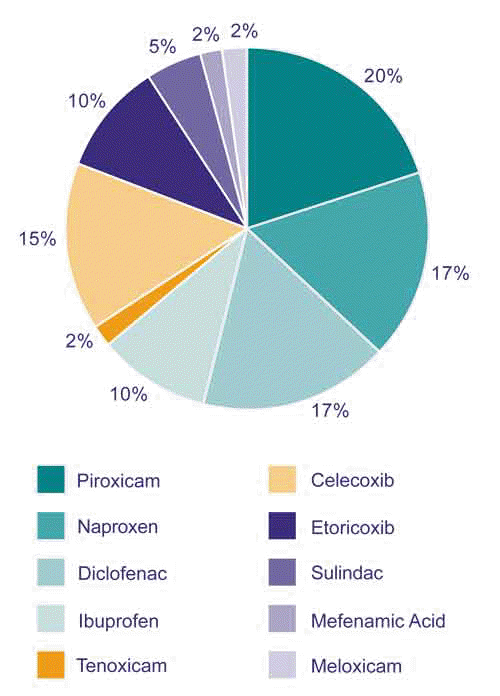
Figure 2: NSAIDs associated with severe cutaneous adverse reactions reported to CARM
SCARs are idiosyncratic and independent of dose or duration of use. People who appear most at risk are older patients, women and those early in the course of therapy. The onset of these reactions generally occurs within the first month of treatment1.
Prescribers should advise patients of the signs and symptoms of SCARs. Patients should be advised to consult their doctor at the first appearance of a skin rash, new onset fever without explanation, mucosal lesions or any sign of hypersensitivity. If a SCAR occurs, NSAID treatment should be discontinued immediately2-4.
References
- Ward KE, Archambault R, Mersfelder TL. 2010. Severe adverse skin reactions to non-steroidal anti-inflammatory drugs: A review of the literature. American Journal of Health-System Pharmacy 67: 206-13.
- Arrow Pharmaceuticals (NZ) Ltd. 2011. Arrowcare Ibuprofen Data Sheet 27 June 2011. URL: www.medsafe.govt.nz/profs/Datasheet/i/IbuprofenArrowcaretab.pdf (Adobe PDF document 42KB) (accessed 21 May 2012).
- Novartis New Zealand Ltd. 2010. Diclofenac Sandoz Data Sheet 18 November 2010. URL: www.medsafe.govt.nz/profs/ Datasheet/d/diclofenacsandoztab.pdf (Adobe PDF document 82KB) (accessed 21 May 2012).
- Mylan New Zealand Ltd. 2009. Noflam Data Sheet 2 February 2009. URL: www.medsafe.govt.nz/profs/Datasheet/n/ Noflamtab.pdf (Adobe PDF document 51KB) (accessed 21 May 2012).





