Published: 8 June 2018
Publications
Using New Zealand Data to Review the Risk of Venous Thromboembolism with Combined Oral Contraceptives
Prescriber Update 39(2): 23-24
June 2018
Venous thromboembolism (VTE) is a rare side effect of combined oral contraceptive
(COC) use. Other risk factors for the development of VTE include age, family
history, prolonged immobility, smoking and being overweight, pregnant or
postpartum. The background rate of VTE in women not taking COCs is around
2 in every 10,000 women. Although COCs are often described as doubling or
trebling the risk of VTE, the absolute risk remains small (Table 1).
Table 1: Venous thromboembolism reporting rates versus expected rates
| Combined oral contraceptive – ethinylestradiol plus:a | Number of reports to CARM 2013–2017 | No. of women taking COCb | VTE reporting rate per 10,000 women over 5 years | VTE expected rate per 10,000 women yearsc,d |
|---|---|---|---|---|
| norethisterone | 3 | 25,375 | 1.2 | 5–7 |
| levonorgesterol | 14 | 123,340 | 1.1 | 5–7 |
| cyproterone | 8 | 37,070 | 2.2 | 9–12 |
| desogestrel | 0 | 3,297 | N/A | 9–12 |
| drospirenone | 4 | N/A | N/A | 9–12 |
a See Table 2 for the funded brands.
b Based on the average of the number of women dispensed prescriptions per year from DataPharm (beta) between 2013 and 2016 2.
c European Medicines Agency. 2014. Benefits of combined hormonal contraceptives continue to outweigh risks. Doc Ref: EMA/35464/2014. URL: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Combined_hormonal_ contraceptives/European_Commission_final_decision/WC500160277.pdf (accessed 24 May 2018).
d European Medicines Agency. 2013. Benefits of Diane 35 and its generics outweigh risks in certain patient groups. Doc Ref: EMA318380/2013. URL: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/cyproterone_ ethinylestradiol_107i/European_Commission_final_decision/WC500147176.pdf (accessed 24 May 2018).
Table 2: Available and funded combined oral contraceptive brands
| Active ingredients | Brand names | Funded brand |
|---|---|---|
| ethinylestradiol/norethisterone | Brevinor, Norimin | Brevinor, Norimin |
| ethinylestradiol/levonorgestrel | Ava (20/30/30ED), Levlen ED, Loette, Microgynon (20ED, 30ED, 50ED) Microlut, Monofeme | Microgynon, Levlen |
| ethinylestradiol/cyproterone* | Diane 35, Estelle 35, Ginet | Ginet |
| ethinylestradiol/desogestrel | Marvelon, Mercilon | Marvelon, Mercilon |
| ethinylestradiol/drospirenone | Yasmin, Yaz | Not funded |
* Contraception is a secondary function not an indication for use.
Numerous studies have investigated the likelihood of a differential risk between COCs. Those containing cyproterone, desogestrel or drospirenone have consistently been associated with a higher risk of VTE than COCs containing levonorgestrel as the progestogen component1 .
Medsafe reviewed the New Zealand spontaneous reporting data (2013–2017) together with dispensing data for funded medicines (2013–2016). In the last five years, 29 cases of VTE were reported to the Centre for Adverse Reactions Monitoring (CARM) in association with COCs. We used data from Ministry of Health’s Pharmaceutical collection, DataPharm (beta)2 , to obtain a frequency estimate (Table 1).
VTE was less likely to be reported with COCs containing levonorgestrel (Ava, Microgynon, Monofeme) and norethisterone (Brevinor, Norimin) than with cyproterone containing products (Diane, Ginet, Estelle). Cyproterone/ethinylestradiol containing products are not primarily indicated for contraception but are included here for completeness. Comparatively few prescriptions for COCs containing desogestrel (Mercilon, Marvelon) were dispensed, and there were no reports of VTE occurring during this time period. We could not estimate the frequency of reports for COCs containing drospirenone (Yasmin, Yaz) as these medicines are not funded. Comparison of the frequency estimate with the expected rate shows that these events are underreported.
Figure 1 shows an analysis of the time from starting the COC to the onset of VTE. Unfortunately, in nearly half of the reported cases, the onset time could not be calculated from the information provided. When this information was provided, in the majority of cases the onset was within the first 12 months of use. However, there were a significant number of cases with longer onset times. Therefore, a high suspicion of VTE should be maintained for all women taking a COC and presenting with symptoms associated with VTE. Women prescribed a COC should be informed about these risks. Medsafe has a consumer leaflet to help with these discussions3 .
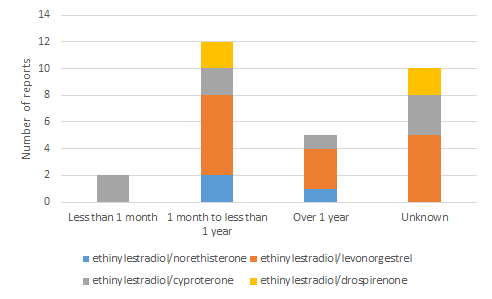
Figure 1: Time to onset of venous thromboembolism associated with use of combined oral contraceptives
Please report any adverse reactions to combined oral contraceptives to CARM (http://nzphvc.otago.ac.nz/report/).
References
- Dragoman M, Tepper N, Fu R, et al. 2018. A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Journal of Gynaecology and Obstetrics 141(3): 287–94. DOI: 10.1002/ijgo.12455.
- Ministry of Health. 2018. DataPharm (beta) version 15 March 2018 (data extracted from Pharmaceutical Collection on 18 December 2017). URL: https://minhealthnz.shinyapps.io/datapharm-beta/ (accessed 16 April 2018).
- Medsafe. 2014. Hormonal contraceptives and blood clots. URL: www.medsafe.govt.nz/consumers/educational-material/Hormonal%20Contraceptives.pdf (accessed 16 April 2018).





