Published: 1 June 2017
Publications
Adverse Reaction Reporting in Older Patients
Prescriber Update 38(2): 18-19
June 2017
Reporting suspected adverse reactions to the Centre for Adverse Reactions Monitoring (CARM) is important for all patients independent of age. It is particularly important for older patients who are more likely to be affected by adverse reactions to medicines due to co-morbidities and reduced renal and hepatic function. Reporting these suspected adverse reactions allows Medsafe and CARM to identify possible safety concerns specific to the elderly.
During the five year period from 2012 to 2016, 17.3% of all cases reported to CARM were in the 65–79 year age group and 5.8% of all cases were in the 80 years and over age group. These proportions have remained consistent over the last five years (Figure 1). Please note that one report may contain multiple medicines and multiple reactions.
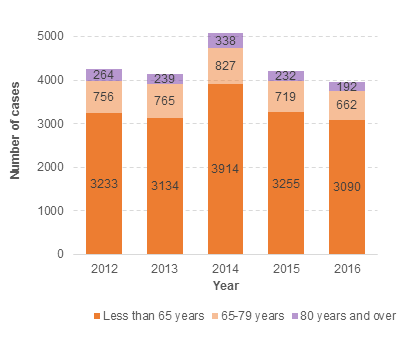
Figure 1: Number of cases of suspected adverse reactions to medicines reported to CARM from 2012 to 2016
Dabigatran was most commonly associated with a suspected adverse reaction reported to CARM in the last five years in both the 65–79 year age group (6.2% of the 3,729 cases) and the 80 years and over group (13.4% of the 1,265 cases) (Table 1). Interestingly, no bleeding reactions were in the top five reactions reported. This is likely due to there being multiple bleeding terms available.
Table 1: Top five medicines reported to CARM in elderly patients in the last five years, 2012–2016
| Age 65–79 | Age 80 & over | |
|---|---|---|
| 1 | Dabigatran | Dabigatran |
| 2 | Iohexol | Zoledronic acid |
| 3 | Influenza vaccine | Influenza vaccine |
| 4 | Zoledronic acid | Clozapine |
| 5 | Clozapine | Omeprazole |
The contrast agent, iohexol was the second most reported medicine associated
with a suspected adverse reaction in the 65–79 year age group (4.9% of cases).
In the 80 years and over age group, the second most reported medicine was
zoledronic acid (5.3% of cases). The influenza vaccine was the third most
reported medicine in both age groups (4.9% of cases in the 65–79 year age
group and 3.6% of cases in the 80 years and over group).
Clozapine is included in the top five medicines reported in both age groups. This is likely due to the monitoring requirements associated with clozapine.
For the five year period, the most commonly reported suspected reaction to a medicine taken by patients in both age groups was rash (Table 2). In the 65-79 year age group, rash was reported in 5.5% of the 3,729 cases. In the 80 years and over age group, rash was reported in 5.4% of the 1,265 cases.
Table 2: Top five reactions reported to CARM in elderly patients in the last five years, 2012–2016
| Age 65–79 | Age 80 & over | |
|---|---|---|
| 1 | Rash | Rash |
| 2 | Nausea | Dyspnoea |
| 3 | Urticaria | Pruritus |
| 4 | Dyspnoea | Nausea |
| 5 | Injection site inflammation | Rash pruritic |
Healthcare professionals are encouraged to report any suspected adverse
reaction(s) to CARM (https://nzphvc.otago.ac.nz/).
Information about how to submit an adverse reaction report can be found
on the Medsafe website (www.medsafe.govt.nz/safety/report-a-problem.asp)
or on the CARM website (https://nzphvc.otago.ac.nz/reporting/).





