Published: December 2012
Publications
Corticosteroids and Musculoskeletal Adverse Events
Prescriber Update 33(4): 37-38
December 2012
Healthcare professionals are reminded that corticosteroids are associated with multiple musculoskeletal adverse reactions including avascular necrosis of the bone, osteoporosis and tendinopathies1.
Avascular Necrosis of the bone
Avascular necrosis of the bone is an uncommon adverse reaction associated with corticosteroids1,2. Higher doses of corticosteroids are associated with a greater risk of avascular necrosis even when used for short periods2. Importantly, avascular necrosis has also been reported with topical application of corticosteroids3.
Osteoporosis
Osteoporosis is a common adverse reaction associated with long-term corticosteroid treatment, where up to 50% of patients are affected1, 4. Bone loss is more rapid during the early stages of therapy, is dose-dependent and primarily occurs in trabecular bone1,4,5. Daily doses of greater than 7.5mg prednisolone (or equivalent) have been associated with a higher risk of fracture than daily doses less than 2.5mg prednisolone (or equivalent)5.
Tendinopathies
Tendinopathies associated with corticosteroid use are predominantly reported in the Achilles and patellar tendons6. Tendon ruptures have also been reported. Tendinopathies have been associated mainly with oral and intra-articular corticosteroid use6.
New Zealand Reports
In New Zealand, 40 reports of musculoskeletal adverse events associated with corticosteroids were reported to the Centre for Adverse Reaction Monitoring (CARM) between January 2000 and June 2012. The majority of the reports were associated with prednisone (30 reports). The remaining reports were associated with dexamethasone (nine reports), triamcinolone (two reports) and methylprednisolone (one report). In two cases, the patient was on more than one corticosteroid. It is worth noting that in all but one case of tendon rupture the patient was also taking a quinolone antibiotic7.
The types of musculoskeletal adverse reactions reported in these 40 reports are shown in Figure 1. Avascular necrosis (55.6%) and osteonecrosis (13.3%) were the most commonly reported musculoskeletal adverse reactions. Of the reported cases of avascular necrosis, two thirds reported avascular necrosis of the femoral head.
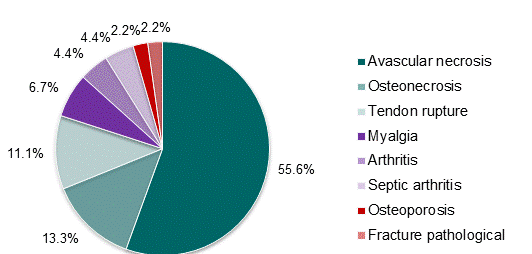
Figure 1: CARM reports of musculoskeletal adverse reactions associated with corticosteroids for the period January 2000 to June 2012
Healthcare professionals are encouraged to educate patients about possible adverse reactions associated with corticosteroid use and to ensure treatment and dose is regularly reviewed. Use of more than one medicine with the potential to cause adverse musculoskeletal effects is likely to increase the risk of an adverse reaction, such as avascular necrosis, osteoporosis, and tendon disorders.
References
- Martindale: The Complete Drug Reference Online. London: Pharmaceutical Press. URL: www.medicinescomplete.com (accessed 14 November 2012).
- Nixon JE. 1984. Early diagnosis and treatment of steroid induced avascular necrosis of bone. British Medical Journal (Clinical Research Edition) 288: 741-4.
- McLean CJ, Lobo RF, Brazier DJ. 1995. Cataracts, glaucoma, and femoral avascular necrosis caused by topical corticosteroid ointment. Lancet 345: 330.
- Lukert BP, Raisz LG. 1990. Glucocorticoid-induced osteoporosis: pathogenesis and management. Annals of Internal Medicine 112: 352-64.
- van Staa TP, Leufkens HG, Abenhaim L, et al. 2000. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 39: 1383-9.
- Blanco I, Krahenbuhl S, Schlienger RG. 2005. Corticosteroid-associated tendinopathies: an analysis of the published literature and spontaneous pharmacovigilance data. Drug Safety 28: 633-43.
- Medsafe. 2012. Quinolones — a tendoncy to rupture. Prescriber Update 33(3): 23-4. URL: www.medsafe.govt.nz/profs/PUArticles/QuinolonesSept2012.htm (accessed 22 November 2012).





