Revised: 6 April 2006
Publications
Statement issued by the Director-General of Health under Section 98 of the Medicines Act 1981 - Unapproved prescription and pharmacist-only medicines from China
Director-General of Health Dr Karen Poutasi is warning women against taking morning after and abortion pills that have been obtained without medical advice and without a prescription, from an unauthorised supplier.
"Consumers can be exposed to substantial health risks if they take medicines, especially prescription medicines, without diagnosis and advice from an appropriately qualified healthcare professional," Dr Poutasi says.
This warning has been issued after surveillance by the Ministry of Health Medicines and Medical Devices Safety Authority, Medsafe, discovered that morning after and abortion pills and oral contraceptives which were not approved for use in New Zealand were being sold to the public.
It appears that the medicines have been imported privately and advertised through a Chinese language website in the last 18 months.
Once aware, Medsafe moved to immediately stop any further supply of these medicines and investigations are continuing into the extent of distribution, Dr Poutasi says.
Medicines are potent substances and must always be used with care.
"Women seeking pregnancy advice should talk to a doctor, pharmacist or authorised prescriber, especially in relation to medication. It is important that women do not take morning after pills, oral contraceptives or abortion medicines that have not been approved for use in New Zealand or are supplied without a prescription or consultation with an accredited healthcare professional," Dr Poutasi says.
Under the law, access to medicines is restricted to protect consumers from the risks medicines can pose if they are not used appropriately or on medical advice. In New Zealand it is illegal to be in possession of a prescription-only medicine without having first obtained a prescription.
Medicines quality, safety and effectiveness cannot be assured unless they are approved for use in New Zealand and are supplied through an authorised supplier or distributor.
"Medicines obtained from unauthorised sources may be dangerous and may even be counterfeit," Dr Poutasi says.
Consultation with a doctor, pharmacist or authorised prescriber will ensure that the risks and benefits of a particular medicine can be adequately considered before it is supplied.
Anyone who has taken a contraceptive medicine, morning-after or abortion pill from a source other than a medical practitioner, pharmacist or authorised supplier and has health concerns should immediately seek advice from a health practitioner, Dr Poutasi says.
Photos of some of the unapproved products
ENDS
For further information contact:
Lucy Taylor
Media Advisor, Ministry of Health
04 496 2067 / 027 207 1406
Questions and answers
What are the medicines found by Medsafe surveillance?
| Product | Description |
|---|---|
| Yu Ting | Contains levonorgestrel 0.75mg. Stated use: Emergency Contraceptive Pill (ECP) |
| Anting | Contains levonorgestrel 0.75mg. Stated use: Emergency Contraceptive Pill (ECP) |
| Si Mi An | Contains Mifepristone 10mg. Stated use: Emergency Contraceptive Pill (ECP) |
| No trade name – labelled as Mifepristone 25mg | Contains: Mifepristone 25mg. Stated use: Pregnancy termination up to 49 days in combination with Misoprostol |
| Mi Suo | Contains: Misoprostol 0.2mg. Stated use: Pregnancy termination up to 49 days in combination with Mifepristone 25mg |
| Marvelon (Chinese version) |
Appears to be Chinese market version of NZ-registered product. Stated use: Oral contraception |
Levonorgestrel 0.75mg – is legally available in New Zealand as Postinor-2
and Levonelle but only on a doctor’s prescription, from an authorised prescriber
or from certain pharmacies.
Mifepristone 10mg and 25mg – is not available in New Zealand in these strengths and is a prescription only medicine.
Misoprostol 0.2mg– is not available in New Zealand for this indication and is a prescription medicine.
Other medicines
Other products have also been found and testing will be required to identify whether they contain active medicinal ingredients.
Where have these medicines come from?
Indications are that they have been imported from China. The medicines are labelled in Chinese characters and appear to have originated from a factory in China.
Adverse effects
The intended use of most of the products involved will mean that they
are likely to have been used immediately after purchase. However, consumers
may have suffered ill effects at the time the medicine was taken and should
consult a medical practitioner if concerned about their health particularly
if pregnancy has continued despite treatment. These medicines may cause
birth defects.
With use of the medicine Levonorgestrel – nausea and abdominal pain are
common side effects. This medicine is not 100% effective and the effectiveness
diminishes if vomiting occurs or if the medicine is not taken promptly.
Close medical supervision is required when the medicine Mifepristone is
taken to terminate a pregnancy. Serious complications may occur such as
infection or haemorrhage. It also commonly causes uterine contractions and
may cause heavy vaginal bleeding. Nausea vomiting and diarrhoea are very
common.
Women taking the medicine Misoprostol may experience adverse effects including
nausea, vomiting, diarrhoea and abdominal pain.
Legal status
These products contain a prescription medicine and are for a therapeutic
use only. They are considered to be medicines distributed without the consent
of the Minister of Health, in contravention of the Medicines Act 1981. Before
the Minister gives consent for the distribution of a medicine, its quality,
safety and efficacy must be assessed to ensure it meets international guidelines.
The medicines in question have not gone through an approval process in New
Zealand.
A medicine includes any substance administered to human beings for a therapeutic
purpose. The meaning of therapeutic purpose includes: treating or preventing
disease, diagnosing disease, effecting contraception, inducing anaesthesia
and altering physical aspects or physiological functions of the body. A
full definition is provided in the Medicines Act 1981 sections 3 and 4.
Have these products been removed from the market?
Yes. Ministry enforcement officers seized supplies of these medicines. There are no other known supplies but the possibility that other distributors may be operating cannot be ruled out.
What should consumers do if they are still taking any of these medicines?
Consumers currently taking any of these products should immediately seek medical advice.
How many people have taken these products in New Zealand?
There is no reliable information about how many people have taken these products.
How were these illegal products being sold?
Information so far indicates that these medicines were sold initially through advertising on a Chinese language website.
General warning for consumers about purchasing products for which therapeutic claims are made through websites
Medicines or products that are being sold through websites or website
contacts for which therapeutic claims are being made may not be legal in
New Zealand. All products for which a therapeutic benefit is being claimed
must first be 'approved' by the Minister of Health before they can be marketed.
Any concerns should be reported to Medsafe. Contact details can be found
on the Medsafe website: www.medsafe.govt.nz
Adverse reactions to any medicinal products should be reported to the Centre
for Adverse Reactions Monitoring
http://nzphvc.otago.ac.nz/report/.
Reporting adverse reactions is key in helping to find illegal medicines
at an early stage.
Important advice to suppliers
Medicines are products sold or supplied principally for a therapeutic
purpose. Selling, distributing or advertising the availability of a medicine
for which consent to distribution has not been given by the Minister of
Health is in breach of section 20(2) of the Medicines Act 1981.
On conviction, the maximum penalty for an individual who sells a medicine
without first having it registered through the regulatory process administered
by Medsafe is $20,000 or up to 6 months in prison. In the case of a body
corporate, the maximum penalty is a fine up to $100,000.
The Ministry of Health advises traders that it takes breaches of the Medicines
Act very seriously where patient or consumer safety is put at risk.
Photos of unapproved prescription and pharmacist-only medicines from China
| Product | Description |
|---|---|
Yu Ting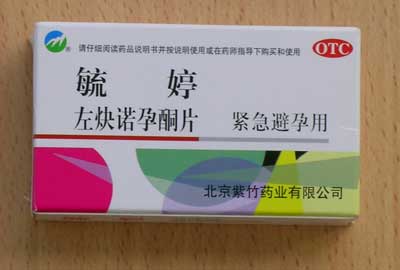 |
Contains levonorgestrel 0.75mg Stated use: Emergency Contraceptive Pill (ECP) |
Anting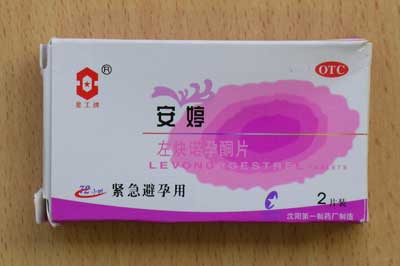 |
Contains levonorgestrel 0.75mg Stated use: Emergency Contraceptive Pill (ECP) |
Si Mi An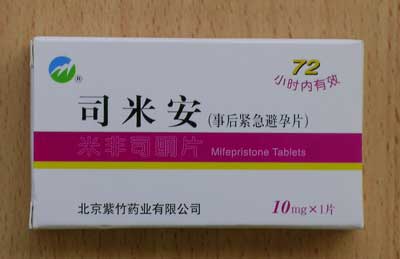 |
Contains Mifepristone 10mg Stated use: Emergency Contraceptive Pill (ECP) |
No trade name – labelled as Mifepristone 25mg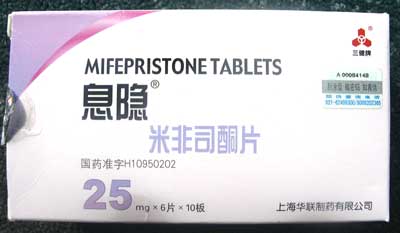 |
Contains: Mifepristone 25mg Stated use: Pregnancy termination up to 49 days in combination with Misoprostol |
Mi Suo |
Contains: Misoprostol 0.2mg Stated use: Pregnancy termination up to 49 days in combination with Mifepristone 25mg |
Marvelon (Chinese version) |
Appears to be Chinese market version of NZ-registered product. Stated use: Oral contraception |





